-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2020; 10(2): 27-29
doi:10.5923/j.ijmc.20201002.03
Received: Nov. 11, 2020; Accepted: Nov. 28, 2020; Published: Nov. 30, 2020

Study of Benzene Vapor Adsortion in Adsorbents Based on Oil Waste
Hudayberganova Nagima Turdibayevna1, Rizaev Abdumalik Nabievich1, Musaev Olimjon Mavlandjanovich2
1Engineering Communications and Systems Department, Construction Faculty, Tashkent State Transport University, Tashkent, Uzbekistan
2Heading Specialist, The Administration of the President of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Hudayberganova Nagima Turdibayevna, Engineering Communications and Systems Department, Construction Faculty, Tashkent State Transport University, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

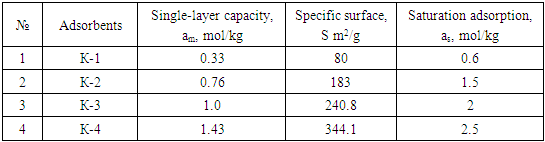
The article studied the optimal conditions for obtaining effective adsorbents on the basis of coke, the adsorption of benzene vapor on activated adsorbents. It was found that benzene vapor in the obtained adsorbents is strongly bound by the formation of ~ 10% adsorbate p complex in K-4 (coke-asphalt) during adsorption.
Keywords: Coke, Benzene, Adsorbent, Absorbent, Adsorption, Desorption, Isotherm, Monolayer capacity, Specific surface
Cite this paper: Hudayberganova Nagima Turdibayevna, Rizaev Abdumalik Nabievich, Musaev Olimjon Mavlandjanovich, Study of Benzene Vapor Adsortion in Adsorbents Based on Oil Waste, International Journal of Materials and Chemistry, Vol. 10 No. 2, 2020, pp. 27-29. doi: 10.5923/j.ijmc.20201002.03.
1. Introduction
- Today, due to the rapid development of industries in the world and the expansion of the application of adsorption processes, the production of cheap adsorbents based on local raw materials and industrial waste is one of the most pressing issues [1]. It is known that activated carbon adsorbents are derived from various carbon raw materials: lignite and coal [2], wood and cellulose [3], peat [4], liquid and gaseous hydrocarbons [5], synthetic polymers [6], plant waste [7]. and other raw materials (waste from construction, bitumen, car tires, polyvinyl chloride and other synthetic polymers).Pyrolysis of wood waste in an oxygen-free environment for the production of activated carbon and its activation using water vapor [8] and orthophosphate acid [9] have also been studied in detail. Currently, the technology of production of carbon adsorbents, which activates natural or synthetic raw materials by steam-gas method, involves two different processes: carbonization of the raw material (pyrolysis) and activation with oxidizing agents at high temperatures, often using water vapor [10]. Carbon dioxide, oxygen and inert gases are also used as oxidizing agents.The cracking process in oil refineries produces large amounts of coke, tar and asphalt. It is possible to obtain effective adsorbents based on coke, tar and asphalt and to treat industrial wastewater using these adsorbents.
2. Materials and Methods
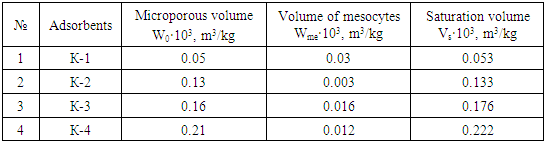
- The coke is pulverized at 10 nm and heated at 800°C for 4 hours in an airless medium. Coke adsorbent (K-1), K-1 is activated by water vapor at 800°C in airless conditions. K-2, tar in a 1: 1 mass ratio to the crushed coke and asphalt (coke-tar, coke-asphalt) were added and quenched for 6 h, then each was activated with water vapor at 400°C for 2 h and at 800°C for 4 h in airless conditions, respectively (K-3, K-4) adsorbents were obtained and conditionally named. The adsorption properties of the obtained adsorbents were studied according to the adsorption of benzene vapor. Benzene obtained as an absorbent was purified under vacuum before use in adsorption, its vapor pressure was frozen until it was the same as the vapor pressure data given in the table for pure benzene, and then dissolved gases were released [11].In activated adsorbents, benzene vapor adsorption isotherms were measured in a McBen-sensitive quartz coil device [12]. Before measuring the adsorption of benzene vapor on adsorbents, each adsorption system was vacuumed to a residual pressure of 1.33·10-3 Pa and heated at 473 K for 8 h after which adsorption isotherms were obtained.
3. Results and Discussion
- From the adsorption isotherms in the studied systems, it was found to be 2.5 times higher in K-2, 3.3 times higher in K-3, and 4.2 times higher in K-4 than in K-1. From the adsorption isotherms in the studied systems, benzene adsorption at K-4 was found to be higher than other adsorbents. In adsorbents, the amount of adsorption was observed to rise sharply from the relative pressure zero to P/Ps≈0.2 and approach the saturation state in the range P/Ps≈0.8-1. The hysteresis in the isotherms formed adsorption rings in the range of specific relative pressure (P/Ps≈0.1–0.2) where the adsorption lines merged with the desorption lines. This suggests that adsorption at higher specific pressures is accompanied by capillary condensation (Figure 1).
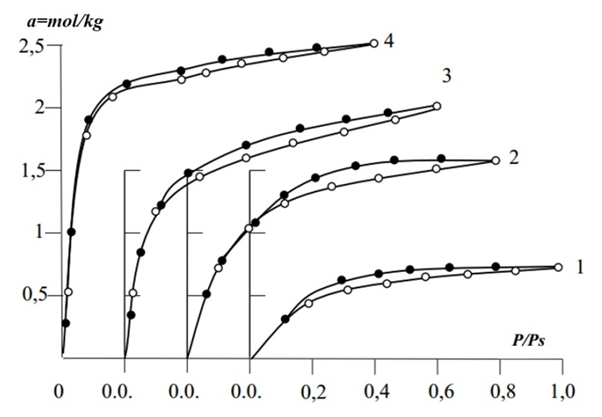 | Figure 1. Benzene vapor adsorption isotherms in K-1 (1), K-2 (2), K-3 (3), K-4 (4) |
|
|
4. Conclusions
- In the adsorbent K-4 obtained on the basis of coke and asphalt, it was found that during the adsorption process ~10% benzene molecules are strongly bound due to the formation of p complexes. In K-4, benzene adsorption is characterized by high adsorbents, cracks between the adsorbent layers, and high porosity compared to other adsorbents. According to the results of benzene adsorption on adsorbents, these adsorbents can be used in various fields, which allows to eliminate to some extent the demand of the Republic for adsorbents.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML