-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2020; 10(2): 23-26
doi:10.5923/j.ijmc.20201002.02
Received: Oct. 14, 2020; Accepted: Oct. 28, 2020; Published: Nov. 5, 2020

Adsorption Isotherm, Differential Heat, Entropy and Thermokinetics of Benzene Vapor in Pakistan Bentonite
Mamajonov Mokhfora Abdulxakimovna1, Salikhanova Dilnoza Saidakbarovna2, Abdurakhmonov Eldor Baratovich2, Ismoilova Mukhtasar Abdumutallib Kizi2
1Basic Doctoral Student of Namangan Institute of Engineering and Technology, Namangan, Uzbekistan
2Institute of General and Inorganic Chemistry of the Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

As a result of changes in the chemical structure of rocks under various influences, clay minerals are formed in nature. Aluminum oxide and silicon oxide serve as the basis of clay minerals. By determining the specific surface area of mineral adsorbents of molecular adsorption in different shapes and structures, the study of the laws of surface chemistry on the basis of the laws awakens a great interest. Based on the energy properties formed during the adsorption of adsorbate molecules, it is possible to determine the structure of the bentonite-forming layers, the migration of cations. Owing to the low charge value and weak electrostatic interaction between the three-layer surface and the multi-layer cations, minerals of the montmorillonite group are capable of multilayer sorption of various substances - cations, water molecules and many organic compounds. Saying differently, the processes of metabolism, absorption and desorption of substances in this group of minerals can occur not only on the outer but also on the inner surfaces of crystallites. The data obtained contribute greatly to the development of the theory of adsorption forces and intermolecular interactions. Determination of adsorption isotherm, differential heat and thermokinetics of benzene in Pakistan bentonite was carried out using adsorption calorimetry method in high vacuum adsorption device. They are based on differential molar entropy and free adsorption energy. The mechanism of benzene adsorption on this bentonite has been fully elucidated. Adsorption isotherms were described using BET and Langmuir equations.
Keywords: Isotherm, Differential heats, Differential entropies, Thermokinetics, Pakistani bentonite, Ammonia, Adsorption calorimetry
Cite this paper: Mamajonov Mokhfora Abdulxakimovna, Salikhanova Dilnoza Saidakbarovna, Abdurakhmonov Eldor Baratovich, Ismoilova Mukhtasar Abdumutallib Kizi, Adsorption Isotherm, Differential Heat, Entropy and Thermokinetics of Benzene Vapor in Pakistan Bentonite, International Journal of Materials and Chemistry, Vol. 10 No. 2, 2020, pp. 23-26. doi: 10.5923/j.ijmc.20201002.02.
Article Outline
1. Introduction
- Clays are mainly composed of microcrystalline particles of a small group of minerals called clay minerals [1]. Clay minerals involve in naturally occurring layered and layered chain silicates, and allow to add layers that are mainly formed during chemical decomposition of rocks, accumulation of sediments, as well as their post-sedimentation, including in the process of hydrothermal action or in other ways [2]. One of the important features of the crystal structure of clay minerals is their active interaction with water.Bentonite clays (bentonites) are considered mineral products adjuncting of the class of aluminoslicates [3]. Originally, bentonites are formed as a result of changes in volcanic rocks or are considered autigenic minerals [4], i.e., minerals of sedimentary rocks are formed as a result of sedimentation or subsequent sedimentation [5]. The most actual mineralogical component of bentonite clays is smectite (clay mineral). For this reason, the great attention is paid to the mineralogical analysis of smectites. Smectite minerals are divided into two subgroups: dioctahedron and trioctahedron [6].Only 2/3 of all octahedral cavities are filled with cations in dioctahedral smectite, and Al3+ cations are mainly located in the center of the octahedrons. Whereas, in trictahedral smectites, all octahedral areas are filled with cations, and Mg2+ cations are located mainly in the center of the octahedron [7].The high absorption capacity of clays composed of minerals of the montmorillonite group is widely used in various fields [9].Vermiculite and montmorillonite have the highest absorption capacity of all clay minerals (80–150 mg-eq/100 g). The high sorption capacity of montmorillonite is associated with the exchange of ions not only on the outer surface of its crystals, but also inside the crystal lattice in the spaces between the silicon-oxygen tetrahedral layers. The mechanism of sorption of water contaminants from clay minerals is very complex and is caused by various chemical effects: hydrogen bonds, ion-dipole and ion-ion interactions, coordination bonds, acid-base reactions, and Van-Der Vaals forces [4,9].
2. Object and Methods of Research
- The differential heat of adsorption was measured on DAK 1-1 calorimeter of a Tean-Calvet model. The volumetric method was used in the determination of adsorption isotherm. The accuracy error of the adsorption isotherm is 0.1% and of the heat is up to 1% [10].Benzene obtained as an adsorbate was purified and dried under vacuum conditions before using in sorption. Dissolved gases were removed until its vapor pressure was the same as the vapor pressure data given in the tables for pure benzene. Then, it was determined to be consistent with the data presented in the literature [11].Benzene adsorption on Pakistan bentonite was carried out at 303 K.
3. Results and Discussions
- In Figure 1, the isotherm of benzene adsorption in Pakistani bentonite is presented in semi-logarithmic coordinates. The initial logarithmic value of the adsorption isotherm is -9.58. In the later stages of benzene adsorption in bentonite, the isothermal lines gradually rise. The logarithmic value of the isotherm of 200 μmol/g of sorbed benzene molecules is -5.5, in which the adsorbates are localized. The inner adsorption layer of 200 μmol/g clay minerals is filled with benzene. When the adsorption reaches 800 μmol, the isotherm value is -1.32. In the subsequent absorption of benzene molecules tends towards the adsorption axis, and when it reaches 1600 μmol/g, benzene reaches saturation levels.
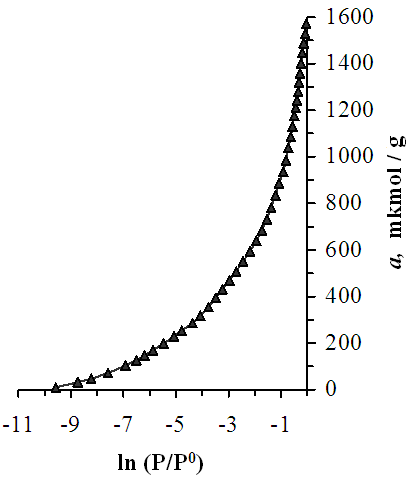 | Figure 1. Benzene vapor adsorption isotherms in Pakistan bentonite at 303 K |
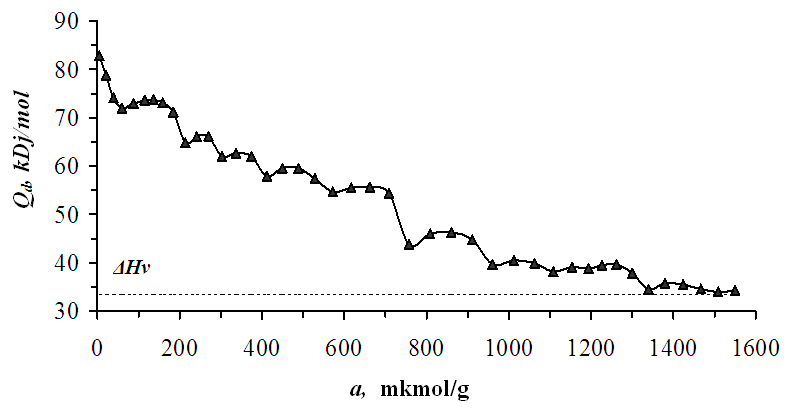 | Figure 2. Differential heat of benzene adsorption on Pakistan bentonite at 303 K. Benzene adsorption at 303 K Horizontal dashed lines - heat condensation |
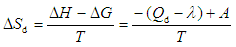 λ- condensation of the heat, ∆H and ∆G- enthalpy and free energy change, Т- temperature, Qd´- avarage differential heat.
λ- condensation of the heat, ∆H and ∆G- enthalpy and free energy change, Т- temperature, Qd´- avarage differential heat.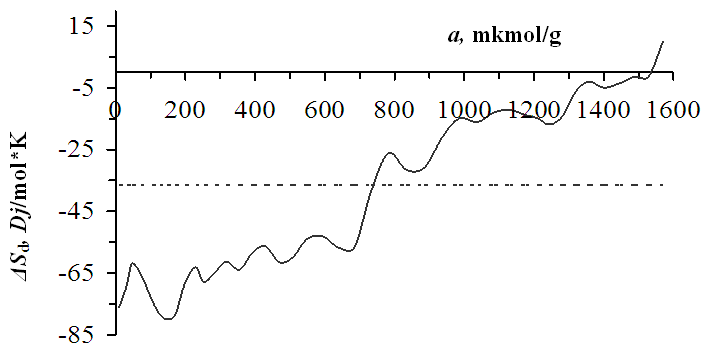 | Figure 3. Differential entropy of benzene adsorption on Pakistan bentonite at 303K temperature. Horizontal bar lines are the mean integral entropy |
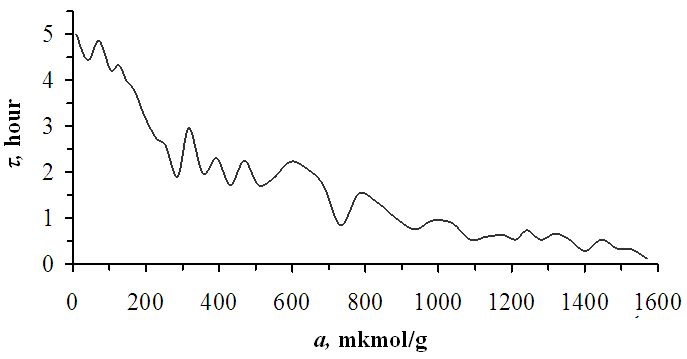 | Figure 4. Equilibrium time of benzene adsorption in Pakistan bentonite at 303 K temperature |
4. Conclusions
- It was found from the low sorption of benzene molecules in the inner layer of bentonite that the number of sorption cells was less than in other layers. The adsorption heat at initial fillings is 82.9 kDj/mol. The differential heat of adsorption decreases in the form of a wavy step. Based on the values of the adsorption isotherms, the specific surface area was determined using the BET and Langmuir equations. The average integral entropy value is -36.5 Dj/mol*K. The adsorption equilibrium time initially starts at 5 hours and decreases to a few minutes depending on the gradual saturation of the bentonite layers.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML