-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2020; 10(2): 17-22
doi:10.5923/j.ijmc.20201002.01
Received: Oct. 5, 2020; Accepted: Oct. 30, 2020; Published: Nov. 5, 2020

Determination of Ammonia’s Adsorption Properties in NaLSX Zeolite by Calorimetric Method
Abdurahmonov Eldor Baratovich1, Rakhmatkarieva Firuza Gayratovna2, Ergashev Oybek Karimovich3
1Candidate of Chemical Sciences (Ph.D.) Institute of General and Inorganic Chemistry of the Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan
2Doctor of Chemical Sciences, Senior Researcher, Institute of General and Inorganic Chemistry of the Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan
3Doctor of Chemical Sciences, Namangan Institute of Engineering and Technology, Vice Rector of Innovation and Scientific Affairs, Namangan, Uzbekistan
Correspondence to: Abdurahmonov Eldor Baratovich, Candidate of Chemical Sciences (Ph.D.) Institute of General and Inorganic Chemistry of the Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The research of the molecules’ adsorption of different geometric shapes and electronic structures together with zeolites of different composition and structure arouses simultaneous interest in the research of the influence of the chemical nature of the surface of adsorbents on adsorption.Zeolites are porous crystals, their adsorption properties are physicochemical constants, therefore, it is theoretically possible to determine the micropore structure of zeolites and to calculate their interaction with the potential interacting energy adsorbate-adsorbate and adsorbate-adsorbent in zeolites. These data are of great interest for the development of the theory of adsorption forces and intermolecular interactions. Nevertheless, the calculation of the potential energy of adsorption is difficult due to the complexity of the crystal structure and the potential field in the zeolite voids. In order to determine the acidity and basicity properties of the composition, it is necessary to study the laws of adsorption of ammonia molecules. In the determination of ammonia adsorption isotherm, differential temperatures and thermokinetics in NaLSX zeolite was carried out using the method of adsorption calorimetry in a high vacuum adsorption device. They are based on differential molar entropy and free adsorption energy. The adsorption isotherm is fully described by the three-dimensional equation of the theory of volumetric micropore occupancy (VMOT). The average molar integral entropy of ammonia molecule adsorption on NaLSX zeolite is 59.64 Dj/mol*K, indicating a strong localization of ammonia molecules in NaLSX zeolite.
Keywords: Isotherm, Differential heats, Differential entropies, Thermokinetics, NaLSX zeolite, Ammonia, Adsorption calorimetry
Cite this paper: Abdurahmonov Eldor Baratovich, Rakhmatkarieva Firuza Gayratovna, Ergashev Oybek Karimovich, Determination of Ammonia’s Adsorption Properties in NaLSX Zeolite by Calorimetric Method, International Journal of Materials and Chemistry, Vol. 10 No. 2, 2020, pp. 17-22. doi: 10.5923/j.ijmc.20201002.01.
Article Outline
1. Introduction
- The technologies for ammonia removal from wastewater are based on physicochemical and biochemical treatment methods [1]. One of these treatment methods is adsorption, which is a low-cost process. Different adsorbents, such as wheat straw biochars, pine sawdust or zeolites, can be effective in adsorbing ammonium in wastewater [2–12].Several studies have reported on the adsorption of ammonium ions by natural or synthetic zeolitic material adsorbents as well [9–12]. Zeolite - aluminosilicate hydrate minerals with a porous, three-dimensional crystal structure are considered an excellent ion-exchange material because of their high selectivity for NH4+ due to their microstructure [2]. An adsorbent of natural zeolites possesses a polar surface and is therefore able to attract ammonium ions quickly and effectively [1]. The removal of ammonia from water was carried out by using natural and synthetic zeolites. In this research three types of natural zeolites (clinoptilolite, mordenite and ferrierite), and synthetic zeolite A were used. The different forms of zeolites such as sodium, potassium and calcium forms were investigated [9]. It was concluded that natural zeolites show high selectivity for ammonium ions with respect to other monovalent ions despite the much higher theoretical exchange capacity of zeolite A. In the study 11, the clinoptilolite was fused with sodium hydroxide prior to a hydrothermal reaction, and it was transformed to a modified zeolite Na–Y. The results were acceptable, showing that modified zeolite Na–Y exhibited a much higher uptake capacity compared with that of clinoptilolite. At an initial concentration of 250 mg/L NH4+, the ammonium ion uptake value of sample 2 was 19.29 mg/g NH4+ adsorbed, while that for sample 1 was only 10.49 mg/g1 NH4+ adsorbed.The emergence of high-energy complexes in the adsorption of NaX zeolite ammonia is dependent on the interaction of ammonia molecules in the SIII' and SII voids in two adjacent states, two abutting sodium cations. SIII' generates heat of adsorption on cations in the void, when the extrapolation of ammonia is Н+ equal to 110 kDj/mol, ~ 90 kDj / mol, relative to the Qd curve to zero saturation [13-14].In our research, the adsorption of ammonia molecules in various synthetic zeolites was carried out by the adsorption microcalorimetric method in a high-vacuum adsorption device, and the full thermodynamic properties of the adsorption processes were described [13-38].
2. Materials and Methods
- The composition of the zeolite obtained for the study is Na96(AlO2)96(SiO2)96. The adsorption-calorimetric tip used in this study allows to reveal the detailed mechanism of adsorption processes occurring in adsorbents and catalysts, as well as obtaining adsorbents and catalysts, moreover, obtaining high-precision molar thermodynamic characteristics.Adsorption measurements and doses of adsorbate were performed using a universal adsorption device, in the working part of which only mercury valves were used and the valves were replaced with vacuum grease [39]. The device allows to dose the adsorbate by both gas-volume and liquid-volume methods. As a calorimeter, a modified DAK 1-1 calorimeter with high accuracy and reliability was used.
3. Result and Discussion
- Ammonia adsorption isotherms to NaLSX zeolite were carried out in a volumetric manner at a temperature of 303 K, Figure 1 provides the results of the experiment (1) and the re-described characteristics (2) using the equation of the volumetric saturation theory of micropores. The isotherm does not change in the same plane as the pressure increases, but rather each of them reflects the transition of one type of centers to another. The isotherm (abscissa) axis consists of a logarithmic (ln), adsorption (N) (N-supervoid and 1/8 NH3 molecule number of elementary cell) (ordinal) axis, which allows us to imagine the adsorption process over the entire pressure equilibrium range. In the adsorption of the three ammonia molecules, the isotherm line goes vertically at the initial filling of the zeolite micropores, where the isotherm ranges from ln(p/p°)=-18 to ln(p/p°)=-16,8, and the adsorption is N=3 C6H6/1/8 u.c. Na + cations in the SIII′ void rise sharply when adsorbed, then turns to the adsorption axis and grows to 6 NH3/1/8 u.c (SI’). In the adsorption after 6 NH3/1/8 u.c, the isotherm 18 NH3/1/8 u.c. increases with a slope to the adsorption axis and is partially saturated (SII).
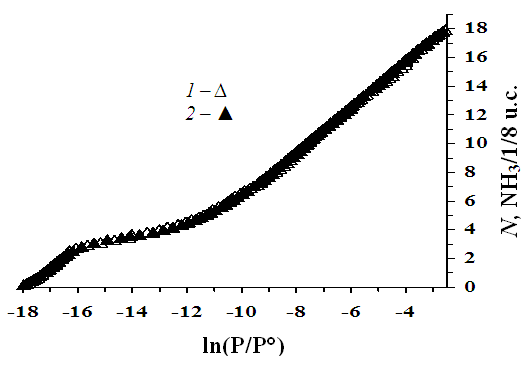 | Figure 1. Isotherm of ammonia adsorption in zeolite NаLSX at 303 K. 1- experimental data 2 – calculated data using the theory of volumetric micropore occupancy (VMOT) |
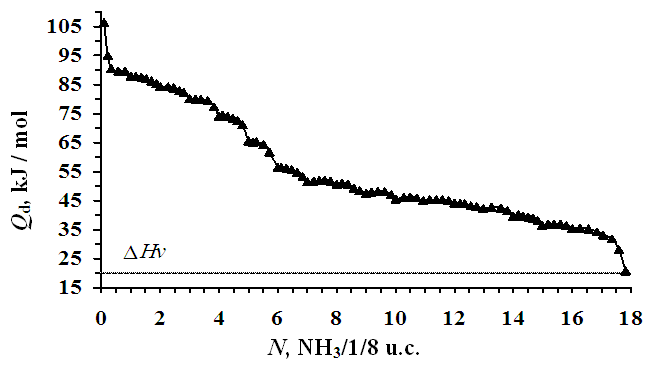 | Figure 2. Differential heats of adsorption of ammonia in zeolite NaLSX at 303 K. Horizontal dotted line - heat of condensation of ammonia at 303 K |
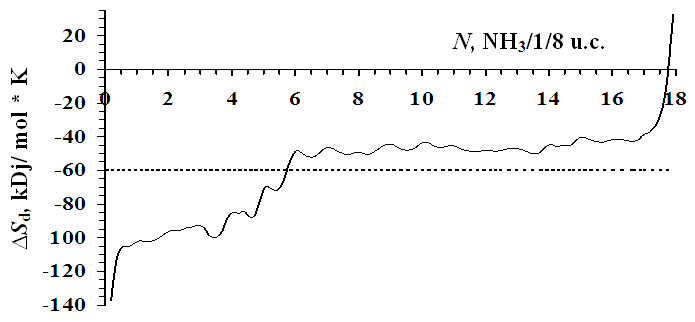 | Figure 3. Differential entropies of adsorption of ammonia in zeolite NaLSX at 303 K. Entropy of liquid ammonia is taken as zero. The horizontal dashed line is the mean integral entropy |
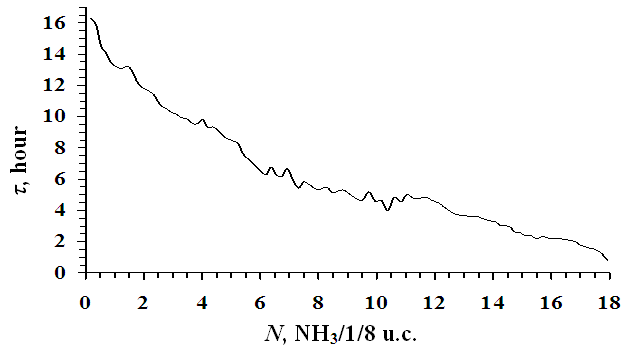 | Figure 4. Time to establish adsorption equilibrium depending on the adsorption of ammonia gases in NaLSX zeolite at 303 K |
4. Conclusions
- At differential temperatures of ammonia adsorption, there are 4 fragments corresponding to the formation of monomeric complexes of ammonia with Na+ cations in position SIII, then SIII, the adsorption process ends with the formation of four-dimensional complexes with cations in position SII. The temperatures of ammonia adsorption with Na+ cations at positions SIII', SI' and SII are 90, 75.5 and 50 kDj/mol, respectively. The adsorption isotherm is fully described by the three-term equation of VMOT). Na+ cations in the SII position form four-dimensional (NH3)4/Na+ complexes in the supervoid. The average integral differential entropy is -59.64 J/mol*K, and the benzene molecules are adsorbed in the zeolite matrix without solid agitation. It takes a long time for the adsorption equilibrium to be established at the initial saturation. As the saturation gradually increases, the adsorption thermokinetics decreases for a few minutes.
ACKNOWLEDGEMENTS
- We would like to thank the non-copyright Yakubov Y.Yu. (PhD), S.B. Lyapin for providing the necessary information in writing the article and for the doctoral students M.K. Kkhokharov and M.S. Khudoiberganov for their assistance in the experimental work. We would like to thank the authors (Rakhmatkarieva F.G., Ergashev O.K.) and the Agency for Science and Technology of the Republic of Uzbekistan for funding a total of 3 fundamental grants.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML