Sobirova Fotima1, Esanov Rakhmat1, Elmurodov Laziz2, Nataliya Vypova3, Alimjon Matchanov4
1PhD Student, Institute of Bioorganic Chemistry Named after Academician A.S.Sadykov, Academy of Sciences of Uzbekistan, Tashkent, Uzbekistan
2Junior Researcher, Institute of Bioorganic Chemistry Named after Academician A.S.Sadykov, Academy of Sciences of Uzbekistan, Tashkent, Uzbekistan
3Candidate of Biological Sciences, Senior Researcher, Institute of Bioorganic Chemistry Named after Academician A.S.Sadykov, Academy of Sciences of Uzbekistan, Tashkent, Uzbekistan
4Doctor of Chemical Sciences, Leading Researcher, Institute of Bioorganic Chemistry Named after Academician A.S.Sadykov, Academy of Sciences of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Sobirova Fotima, PhD Student, Institute of Bioorganic Chemistry Named after Academician A.S.Sadykov, Academy of Sciences of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The data on the synthesis of lagochiline succinates with succinic anhydride is presented in the article. The structure of the resulting compound was found out by physicochemical methods of analysis, mass spectrometry data and a calculation method. The purpose of this work was to synthesize a number of succinates of the diterpenoid lagochilin and obtain water-soluble salts, as well as to study the physicochemical and spectral properties and structure of the synthesized compounds by mass spectrometry, 1H NMR and a calculation method. To establish the chemical structure of the synthesized compounds, the method of ultra high performance liquid chromatography-mass spectrometry was applied (HPLS-MС/MС).To establish the chemical structure of the synthesized compounds, the method of ultra high performance liquid chromatography-mass spectrometry was applied (HPLS-MС/MС).
Keywords:
Lagochilus Inebrians, Lagochilin, Succinic acid, Succinate, Toxicity, Vascular-platelet hemostasis
Cite this paper: Sobirova Fotima, Esanov Rakhmat, Elmurodov Laziz, Nataliya Vypova, Alimjon Matchanov, Synthesis and Biological Activity of Succinates 3,15,16,18 Tetrahydroxy 9-13-Epoxylabdane, International Journal of Materials and Chemistry, Vol. 10 No. 1, 2020, pp. 8-15. doi: 10.5923/j.ijmc.20201001.03.
1. Introduction
Plants of the genus Lagochilus are known as valuable hemostatic means and are among the most famous medicinal plants of the East. Lagochilus-based drugs are successfully used to stop various bleeding. The main active ingredient is four atomic alcohol, diterpenoid lagochilin, and on its basis a number of acetyl and isopropylidene derivatives were obtained and it was shown that hemostatic activity depends in a certain way on the number of free hydroxyl groups of lagochilin and its derivatives [1-5].Hence, the synthesis of water-soluble derivatives of lagochilin is a promising direction, while assuming the possible enhancement of their water-solubility and hemostatic activity.This can be accomplished by chemical modification of lagochilin at hydroxyl groups, obtaining esters or succinates with diacid anhydrides. One of such objects is succinic acid.Succinic acid (butanedioic acid, ethane-1,2-dicarboxylic acid) is a dibasic saturated carboxylic acid, which is contained in small quantities in many plants as amber. It is illustrated that it stimulates the growth and increases the productivity of plants, accelerates their development. Salts and esters of succinic acid are called succinates (succinum — amber) [6].A method for the synthesis of hemisuccinates, hemiphthalates, acetylsalicylates, cinnamates and p-methoxycinnamates of lupeol, betulin and its 3-O-acetate with anhydrides and acid chlorides is shown in the literature [7-11].It is also demonstrated that the antioxidant activity of succinates is comparable to the synthetic antioxidant ionol [12]. Experimental use of monoethyl ester of succinic acid in rats with streptozotocin-induced diabetes led to a significant decrease in the level of lipid peroxidation products (LPO) in the blood [13-14].In such way, the abovementioned shows the relevance of studies in the field of chemistry for the synthesis of succinates, including those with natural diterpenoids.
2. Materials and Methods
Thin layer chromatographyon SILUFOL plates was used for identification. Thin layer chromatography solvent systems: I benzene: acetone 1: 1. Iodine vapor was used as a developer. For column chromatography, we used silica gel with a particle size of 100/160. IR spectra were recorded on a Perkin-Elmer System-2000 IR-Fourier spectrometer on KBr tablets. HPLS analyzes were carried out on an Ultimate 3000 chromatograph, Thermo Fisher Scientific (USA) equipped with an automatic sampler, a Hypersil GOLD aQ column 100 mm x 2.1 mm, 1.9 μm.General method for the synthesis of lagochiline succinates. A mixture of 1 mmol of lagochiline (3,15,16,18-tetrahydroxy 9-13-epoxylabdane) and excess succinic anhydride was dissolved in 20 ml of anhydrous pyridine. The mixture was boiled under reflux for 14-15 hours. After that, pyridine was evaporated in vacuo. The residue was treated with a 5% solution of cold hydrochloric acid and extracted 3-5 times with benzene, dried with anhydrous sodium sulfate and evaporated in vacuo. The residue was separated by column chromatography. The yield of succinate compounds of lagochilin was 75-90%. Lagochilin from the plant Lagochilus Inebrians was extracted by anhydrous dichloroethane extraction and purified by recrystallization from acetone. The physicochemical properties and the degree of purity of lagochiline were determined by the melting temperature, Rf. The crystal structure of lagochiline was studied by powder diffractometry method [15] and it was shown that it consists of a monohydrate. For the synthesis of succinates, lagochiline monohydrate was used and the method is described in the literature [6]. The general scheme for the synthesis of lagochiline succinate is illustrated in Scheme 1.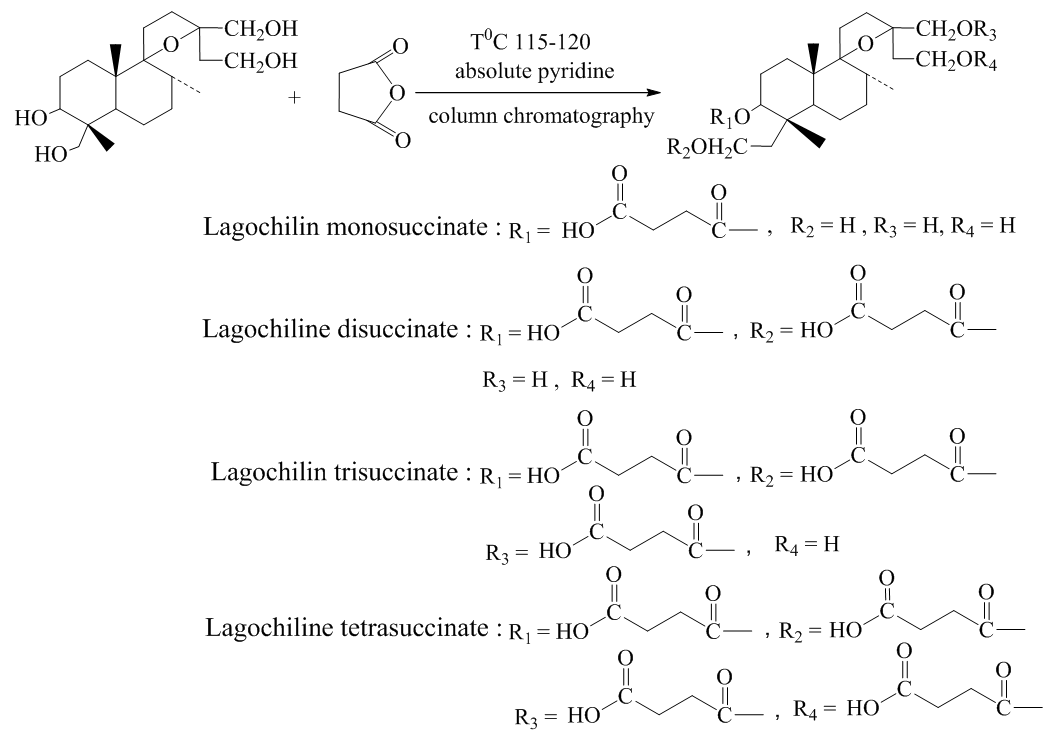 | Scheme 1. Synthesis of succinates 3,15,16,18-tetrahydroxy 9-13-epoxylabdane |
To obtain water-soluble sodium salts of the synthesized succinate compounds, they were dissolved in an anhydrous organic solvent and exposed to an alcoholic solution of sodium hydroxide.
3. Results and Discussion
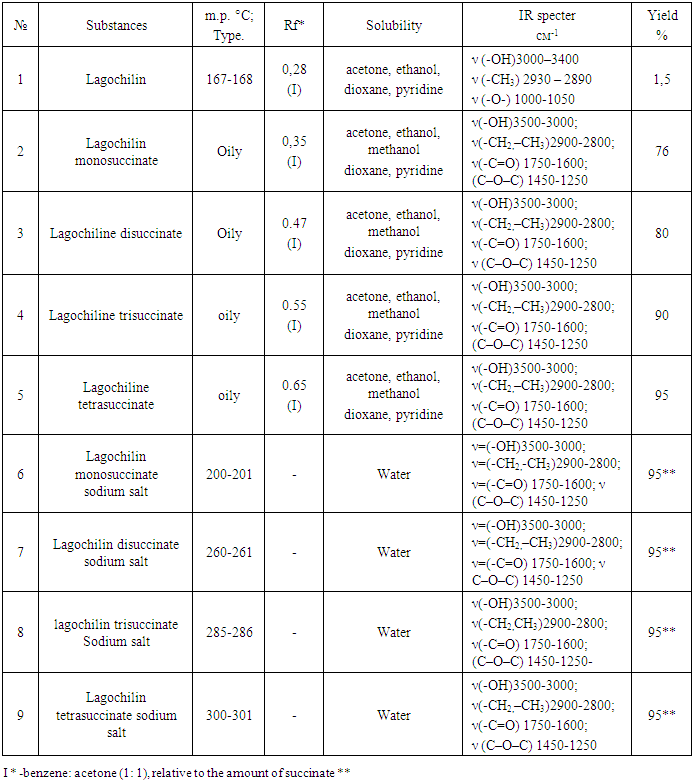
The succinates obtained were identified by the method of thin layer chromatography on Silufol plates in a solvent system: benzene: acetone (1: 1), relative to the initial components. The separation of the final product was carried out by the method of column chromatography in the benzene: acetone system (90:10; 80:20; 75:25; 50:50;). Silicagel brand 160/100 was used. Obtained succinate of lagochilin is characterized by physicochemical and spectral data, which are shown in Table 1.Table 1. Some physicochemical characteristics of lagochiline succinates
 |
| |
|
Lagohilin and synthesized succinates in their structure do not have chromophore groups that could absorb light. Therefore, they cannot be investigated by UV spectroscopy. But they have objective groups that show intense vibrational spectra in the infrared region.The IR spectrum of the synthesized compounds shows characteristic stretching vibration bands at νОH-3800-3600 cm-1 of free hydroxyl groups of lagochilin (3,15,16,18-tetrahydroxy 9-13-epoxylabdane), and the appearance of stretching vibrations at ν=(С = О) -1720- 1600 cm-1 shows that the new substance has a -C = O group, which corresponds to the carbonyl groups of lagochiline succinate. And also bending vibrations ν=(CH2) -3095-3075 cm-1. Stretching vibrations of the ν=СO -1450-1250 cm-1 (-C-O-C-) group are also observed.There is a number of methods for studying the chemical structure of a synthesized compound, such as 1H NMR spectroscopy and mass spectrometry.Figure 1. In the 1H NMR spectra of the synthesized succinates in the range 10.12 - 10.87 m.m the resonance signals of the protons of the hydroxyl group are observed in the form of broadened singlets. The proton signals of the methyl groups of lagochiline (3,15,16,18-tetrahydroxy 9-13-epoxylabdane) are observed in the strong field region within 0.90 - 1.81 m.m.Table 2
 |
| |
|
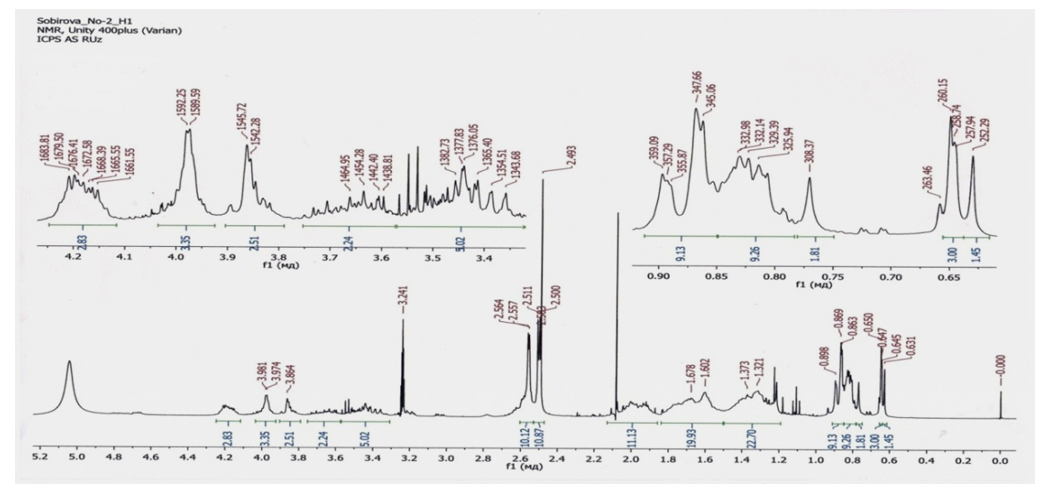 | Figure 1. 1H NMR spectrum of lagochilin tetrasuccinate |
HPLS analyzes were carried out using an Ultimate 3000 chromatograph, Thermo Fisher Scientific (USA) equipped with an automatic sampler, a Hypersil GOLD aQ column 100 mm x 2.1 mm, 1.9 μm; ultrapure water acidified with 0.01% formic acid was used as a mobile phase (A) and acetonitrile (B) at a flow rate of 0.1 ml / min. Chromatographic conditions were selected as follows:Mobile phase (in a gradient with acetonitrile):0.5 minutes 0% B; 0.5-10 minutes 60% B; 10-12 min. 85% B; 12-13 min. 90% B; 13-14 minutes 0% B; The column temperature was set to 35°C.Electron Impact Ionization Mass Spectrometry (HESI-II) was performed under the following conditions: Spray voltage - 4000 V, with monitoring of negative ions;Evaporator temperature - 250°C, Shell gas pressure - 20 psi,Auxiliary gas pressure - 10 psi; Capillary temperature - 100°C; Collision pressure - 1.5 mTorr; The ionization energy is 20 eV.It can be seen from the data presented that the main molecular ion M-m / z 455- corresponds to the hemisuccinate of lagochiline. The next molecular ion m / z 116.9 corresponds to the cleaved succinic acid. Molecular ion m / z 401 corresponds to the remainder of the main ion of the separated three water molecules. It is known from the literature that acetylation of lagochilin passes through the hydroxyl group of the 3 carbon atom. Therefore, we assume that lagochiline monosuccinate is formed at the third carbon atom. The molecular ion m / z 279- corresponds to the lagochilin molecule by cleavage of one water molecule and two hydroxymethyl groups at the 15 and 16 carbon atoms of the lagochilin molecule. The molecular breakdown of lagochiline monosuccinate is shown in Figure 2.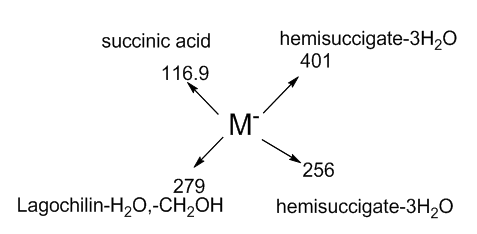 | Figure 2. Molecular decomposition of sodium salt of lagochilin succinate |
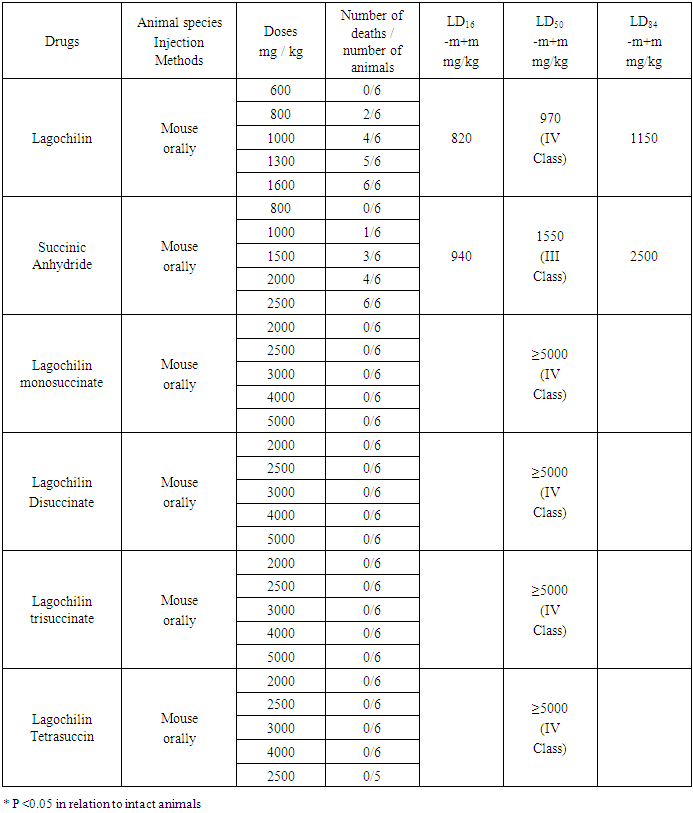
The literature contains data on the molecular breakdown of the lagochilin molecule. By the method of mass spectrometry, the position of the monoacetyl group of lagochiline was established, that it should be located at C-3 or C-18 of the molecule. However, the final location of the acetyl group was established using the PMR spectra. The PMR spectrum (-scale HMDS, CHCl3) shows a three-proton singlet of one acetyl group at 1.98 ppm. and a one-proton quartet at 4.78 ppm, characteristic of the proton at C-3, to which the acetyl group is bound. The signals of protons at C-15, C-16, and C-18 are observed, as expected, in a different region of the spectrum, in comparison with the signals of the corresponding protons in the PMR spectrum of lagochiline tetraacetate. On the basis of these data, the structure of lagochiline 3-monoacetate was proposed for the isolated substance [16]. Therefore, we assume that lagochiline monosuccinate is formed at the third carbon atom. These data were also confirmed by the calculation method. In the Chem Bio Draw Ultra 12.0 program, the minimum energy of formation of lagochilin monosuccinate was calculated for various positions (3,15,16,18). As a result, the following series C3 → C18 → C15 → C16 was obtained, respectively 71.6 kcal / mol → 167.4 kcal / mol → 171.2 kcal / mol → 173.2 kcal / mol. From the calculation data it can be seen that the most optimal substitution is in position 3.Further, acute toxicity was investigated in mice after oral injection in a comparative aspect with the original Lagochilin, with succinic anhydride, which is a part of the studied drugs, as well as the effect on vascular platelet hemostasis in the test for bleeding time and the amount of blood loss in rats in comparison with the drug Glilagin.The experiments were carried out on 126 male rats weighing 165 ± 15 g, 6 animals per group (21 groups). The effect of Lagochilin derivatives on vascular-platelet hemostasis after intragastric injection in various doses was judged by the time of bleeding and the amount of blood loss in rats.The drugs were injected orally in the following doses: mono-, di- and trisuccinates of lagochilin - in doses of 0.5, 1.0, 2.0 and 5.0 mg / kg; tetrasuccinate - in doses of 1.0, 2.5, 5.0 and 8.0 mg / kg, the reference drug substance Glilagin - in doses of 1.0, 2.5, 5.0 and 8.0 mg / kg. Intact animals were used as controls. Then the animals were placed in a thermostat with an open door at a temperature of + 300 C. The animals were kept at this temperature for at least an hour. Then the tip of the tail, about 10-12 mm long, was cut off with sharp scissors. A sheet of filter paper was brought to the tail stump. The paper was pre-weighed. The blood flowing from the tail was evenly distributed on the filter paper. The paper was dried and weighed. The duration of bleeding was recorded using a stopwatch from the moment the first drop of blood appeared until the complete cessation of bleeding. The amount of blood loss was assessed by the weight of the leaked blood in grams [16,17].The study of acute toxicity of the drug was carried out on white mice, males, weighing 20 ± 2.0 g, 6 animals in each group, 210 mice were used in total.Substances and doses:- lagochilin dissolved in 25% ethanol was injected orally at doses of 600, 800, 1000, 1300 and 1600 mg / kg, in the form of a 10% solution;- succinic anhydride was injected orally at doses of 800, 1000, 1500, 2000 and 2500 mg / kg, in the form of a 10% solution;- lagochilin monosuccinate was injected orally at doses of 2000, 2500, 3000, 4000 and 5000 mg / kg, in the form of a 20% solution;The animals were monitored hourly during the first day of the experiment in a laboratory, while survival during the experiment, general condition, possible convulsions and death were used as indicators of the functional state of the animals. Then, every day, for 2 weeks in a vivarium, the animals of all groups were monitored for the general condition and activity, behavioral features, the frequency and depth of respiratory movements, the condition of the hair and skin, the position of the tail, the amount and consistency of fecal masses, the frequency urination, change in body weight and other indicators. All experimental animals were kept under the same conditions and on a common diet with free access to water and food [18,19]. At the end of the experiment, the average lethal dose (LD50) was calculated and the toxicity class was determined [20,21].The obtained data were subjected to statistical processing using the Origin 8.0 application package (USA) with the calculation of the arithmetic mean (M), the mean error of the arithmetic mean (m), Student's test (t) with the calculation of the error probability (P). The significance level of P <0.05 was taken as statistically significant changes.The general behavior of animals after oral injection of Lagochilin to mice at doses of 600-1600 mg / kg was manifested in a decrease in motor activity, slow breathing and slowing of the heartbeat, clustering. The death of animals at a dose of 600 mg / kg was not recorded (0/6). The death of animals in increasing doses was: dose 800mg / kg - 2/6, dose 1000mg / kg - 4/6, dose 1300mg / kg - 5/6, dose 1600mg / kg - 6/6. The death of animals occurred within 1-2 days after injection. As a result, the average lethal dose of Lagochilin when injected orally to mice was LD50 = 970 mg / kg, toxicity class IV (low-toxic compounds).After oral injection of succinic anhydride to mice at a dose of 800 mg / kg, a decrease in motor activity, slow breathing, slowing of the heartbeat, clustering was observed after 15-20 minutes. The behavior of the animals returned to normal after 1-1.5 hours. No deaths were reported (0/6). When the drug was injected at a dose of 1000 mg / kg, a decrease in motor activity, slow breathing and slowing of the heartbeat, clustering was observed after 15-20 minutes, the animals made a faint groaning sound. The behavior of the animals returned to normal after 2-2.5 hours. The death of one animal was recorded after five days (1/6). With the insertion of a dose of 1500 mg / kg after 10-15 minutes, a decrease in motor activity, tachycardia was observed, in some animals, convulsions, a weak groaning sound and clustering were observed. One hour later, one of six mice (1/6) died. The behavior of the other animals returned to normal after 5-6 hours. On the fifth and sixth days, 2 mice died (3/6). At a dose of 2000 mg / kg after 5-10 minutes, a decrease in motor activity, slow breathing, slow heartbeat, reflex vomiting, distended abdomen, and a faint groaning sound were observed in all animals. Position of animals: stand upright on the cage wall. After 30 minutes, convulsions appeared. After 2-4 hours, three mice died, and another day later (4/6). The behavior of the other animals returned to normal in a day. At a dose of 2500 mg / kg, all animals showed a decrease in motor activity, slow breathing, slow heart rate, convulsions, vomiting, distended abdomen, and a faint moaning sound. Also, cramps were observed in animals, which indicates a possible severe pain in the abdomen. After 2-3 hours, 4 mice died and during the day another 2 (6/6). The average lethal dose of succinic anhydride when injected orally to mice was LD50 = 1550 mg / kg, toxicity class IV (low-toxic compounds). After oral injection of monosuccinate Lagochilin to mice at doses of 2000 - 3000 mg / kg, the behavior of the animals did not differ from the norm, at doses of 4000 and 5000 mg / kg, a decrease in locomotor activity, tachycardia, slow breathing, clustering was observed after 10 minutes. The behavior of the animals returned to normal after 1 hour. The death of animals within 14 days was not recorded (0/6). The average lethal dose of LD50 after oral injection in mice for Lagochilin monosuccinate was more than 5000 mg / kg, toxicity class V (practically non-toxic compounds).The hemostatic activity of the new derivatives was investigated by the bleeding time and the amount of blood loss in rats. This test reflects the vascular-platelet mechanism of hemostasis and is determined by the number and state of platelets (their ability to adhere and aggregate).Table 3. Results of acute toxicity indices after oral injection of lagochilin, succinic anhydride, lagohilin derivatives
 |
| |
|
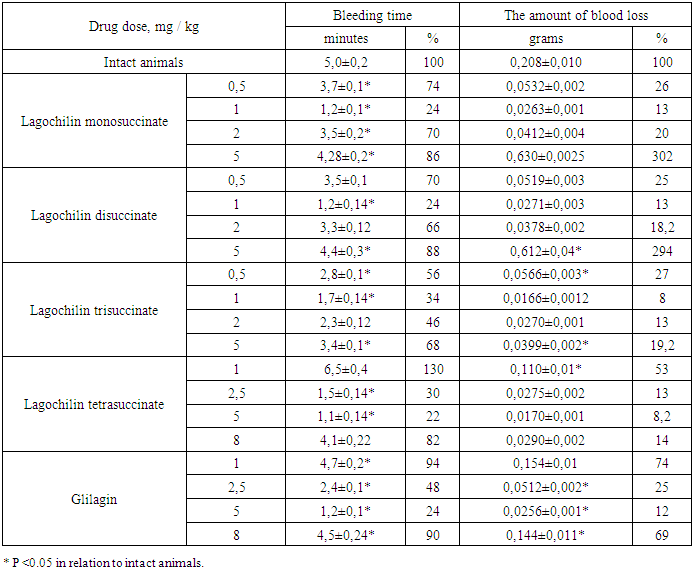
As can be seen from the data in Table 3, the bleeding time after 60 minutes decreased after the injection of Lagochilin monosuccinate at doses of 0.5, 1.0, 2.0 and 5.0 mg / kg, by 1.2-4.2 times. The maximum effect was observed at a dose of 1.0 mg / kg, where the bleeding time was reduced by 4.2 times.Table 4. Bleeding time and blood loss in rats 60 minutes after oral injection of lagochiline derivatives and Glilagin substances (M ± m n = 6)
 |
| |
|
As can be seen from Table 4, the therapeutic index of the studied drugs decreases in the series of Lagohilin succinates = <Glilagin substance <Lagochilin.The introduction of succinic anhydride residues into the Lagochilin structure leads to a significant increase in the therapeutic index by 25 due to a 5-fold decrease in toxicity and a 5-fold decrease in the therapeutic dose.With an equal therapeutic index for Lagochilin derivatives: Lagochilin monosuccinate, the latter has a more pronounced decrease in the amount of blood loss, 1.5 times lower than that of the first two derivatives.
4. Conclusions
1. For the first time was synthesized -mono, -di, -three, tetrasuccinate lagochilin. Some physicochemical and spectral characteristics of the synthesized compound have been studied. The chemical structure is proved by the data of methods of IR spectroscopy, gas chromatography-mass spectrometry, 1Н NMR and calculation method.2. The acute toxicity of the new derivatives of lagochilin was 3-5 times less than their initial components (succinic anhydride and lagoсhilin).3. The most effective dose for succinates of lagochilin was a dose of 1 mg / kg, which reduced the bleeding time in rats by 3.0-4.5 times as compared to intact animals.
References
| [1] | Flora of the USSR. M., L.: AN SSSR. 1954. |
| [2] | M.M. Abramov., S.A. Yaparova. // Obtaining the main active principle from the intoxicating Lagohilus // Jurn. app. chemistry. 1963. T36. No. 11. p. 2554-2556. |
| [3] | U. N. Zainutdinov, R. Islamov, D. N. Dalimov, T. R. Abdurakhmanov, A. D. Matchanov., N. L. Vypova // Structure-activity relationship for hemostatic lagochilin diterpenoids // 38 pp. 161-163. 2002, vol. |
| [4] | N.S. Gulyamova, G.Yu Akmalova., A.Kh. Islomov., U.N. Zainutdinov., G. Rakhmanberdiev. // Technology of production of Lagochirzin and its sodium salt (lagoden) // Chemistry and Chemical Technology. Tashkent. 2010. No. 2, P. 35-38. |
| [5] | U.N. Zaynutdinov., R. Islomov, D.N. Dalimov., T.R Abdurakhmonov., A.D Matchanov., N.L. Vypova //Structure-activity relationship for hemostatic lagochilin diterpenoids// 38 pp. 161-163. 2002, vol. |
| [6] | N.S. Zefirov (chief editor) and others - M. //Chemical encyclopedia. In 5 t. T. 5. Tri-Yatr // Great Russian Encyclopedia, 1998. -- 783 p. |
| [7] | O.B. Flekhter, L.T. Karachurina, V.V. Poroikov, L.R. Nigmatullina, L.A. Baltina, F.S. Zarudiy. V.A. Davydova, L.V. Spirikhin, I.P. Baikova, F.Z. Galin, G.A. Tolstikov. //Synthesis of lupane group triterpenoid esters and their hepatoprotective activity. Bioorganic chemistry. //2000. v. 26. Number 3. from 215-225. |
| [8] | A.C. Ariza., P.MT. Deen., J.H. Robben //The succinate receptor as a novel therapeutic target for oxidative and metabolic stressrelated conditions. Front Endocrinol (Lausanne) //2012; 3 (22): R. 1-8. |
| [9] | Kushnir MM, Komaromy-Hiller G, Shushan B et al. Analysis of dicarboxylic acids by tandem mass spectrometry. High throughput quantitative measurement of methylmalonic acid in serum, plasma, and urine. Clin Chem //2001; 47 (11): R. 1993-2002. |
| [10] | I.A. Telichkin., Robert Koch. Wedge Honey. //1996; (1): S. 78-79. |
| [11] | V.V. Afanasyev. // Clinical pharmacology of Reamberin (outline). Polisan, SPb., // 2005; P. 39. |
| [12] | N.N. Andreeva // Experimental and clinical aspects of the use of Mexidol in hypoxia. Almanac Med// 2009; 4 (9): pp. 193-197. |
| [13] | R. Saravanan, L. Pari. //Succinic acid monoethyl ester, a novel insulinotropic agent: effect on lipid composition and lipid peroxidation in streptozotocin-nicotinamide induced type 2 diabetic rats. Mol Cell Biochem //2007; 296(1-2): Р. 165-176. |
| [14] | L. Tretter., G. Szabados., A. Ando., I. Horvath. // Effect of succinate on mitochondrial lipid peroxidation. 2. The protective effect of succinate against functional and structural changes induced by lipid peroxidation. J Bioenerg Biomembr //1987; 19(1): Р. 31-44. |
| [15] | Z.I. Mavlankulova, U.N. Zainutdinov, S.I. Mukhamedkhanova. VB Leontiev, Kh.A. Aslanov. // 13C NMR spectra of lagochiline and its derivatives. // Chem. natural connect. 1979. No. 1. S. 41-43. |
| [16] | I.E. Akopova //Problems of blood coagulation and hemostasis, Scientific. Proceedings, t XXXIII ed. //1971. from. 13-15. |
| [17] | Grif and K //Guidelines for the study of drugs that affect hemostasis (Chapter 27) Guidelines for conducting preclinical studies of drugs. Part one M.: //2012. - pp. 453-479. |
| [18] | RU. Khabrieva//Guidelines for experimental (preclinical) study of new pharmacological substances, Methods of pharmacological preclinical researched., M. // 2005. S. 45-46. |
| [19] | Grif and K //Methodical recommendations for the study of the general toxic effect of medicinal products (Chapter 1). Guidelines for conducting preclinical studies of medicinal products. Part one M // 2012 - p. 15-17. |
| [20] | M.L. Belenkiy. //Elements of a quantitative assessment of the pharmacological effect. State publishing house of medical literature, // 1963. - 152 p. |
| [21] | K.K. Sidirov// Toxicology of new industrial chemicals. Classification of substances by toxicity. M: Medicine Issue 3. //1973. 47s. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


