-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2020; 10(1): 1-4
doi:10.5923/j.ijmc.20201001.01

Analysis of Specific Surfaces of Composite Material: Clays of Madagascar and TiO2
Andrianainarivelo Mahandrimanana
Department Process and Industrial Ecology, Lecturer at Sciences’ Faculty of Université of Antananarivo
Correspondence to: Andrianainarivelo Mahandrimanana , Department Process and Industrial Ecology, Lecturer at Sciences’ Faculty of Université of Antananarivo.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

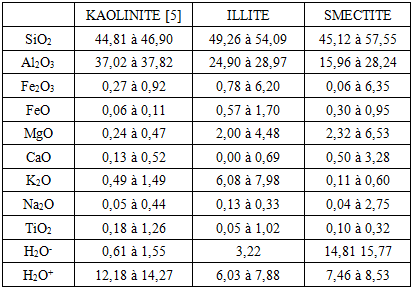
The objective of this study to show that TiO2 can be attached to the external surface of clays. Two types of clay, white and green clay, have been used because those raw materials are very abundant in Madagascar. The composite material is useful to eliminate pathogenic microorganism and decompose organic compounds in polluted water, for example. The BET (Brunauer, Elmett and Teller) analysis is the best method to evaluate the capacity of the composite to catalyze the reaction of decomposition. The BET analysis of specific area are 60.71 m2/g for white clay (micropore surface 9.63 m2/g and outer surface 51.08 m2/g with pore size 151.46 Å, an A° = 1millionth of a millimeter) and 249.52 m2/g for green clay (micropore area 20.51 m2/g and outer surface 229.01 m2/g with pore size 49.96 Å). The size of the micropores is less than 17 Å. When TiO2-clays are treated at 600°C, the specific area of white clay increases, it becomes 94.07 m2/g while pore size decreases (81.39 Å). For green clay, the specific area is about the same (247.61 m2/g) and pore size doesn’t change (49.97 Å).
Keywords: Clay, TiO2, Specific surface, Nanotechnology, Composite material
Cite this paper: Andrianainarivelo Mahandrimanana , Analysis of Specific Surfaces of Composite Material: Clays of Madagascar and TiO2, International Journal of Materials and Chemistry, Vol. 10 No. 1, 2020, pp. 1-4. doi: 10.5923/j.ijmc.20201001.01.
Article Outline
1. Introduction
- Around two thirds of the Malagasy population have no access to drinking water throughout the country [1]. In rural areas, this proportion is more than two out of three people. Just over half of the population has access to sanitation [2]. The situation in schools is even more critical: 79% of primary schools in rural areas do not have water points in their enclosures, 35% do not have latrines and only about 30% of existing infrastructure meets the standards. Given these statistics, the problem of access to safe drinking water remains a challenge for the country. The rural population (between 75% and 80% of the Malagasy population) draws water from places that are not very clean. The source of the high prevalence of diarrhoea diseases comes from these dirty waters (well water, river water...). There are currently different types of wastewater in Madagascar: domestic wastewater, industrial wastewater, stormwater and agricultura wastewater. Most of that wastewater is discharged untreated into the wild despite their high charges as organic and mineral pollutants. Given these circumstances, the availability of a treatment device that is accessible to everyone is necessary. Indeed, the use of local products such as clay could be beneficial to the population.So TiO2-clays seem to be the best solution to clean waste water. Clays are very abundant raw materials in Madagascar. TiO2 can be synthesized by hydrolytic sol-gel process and it’s the best material to eliminate pathogenic microorganism and to decompose organic compounds when it’s exposed under the sun.The ability of clay fixation is linked to the existence of the charge, and to the specific surface or surface of exchange. By their plastic viscosity, clays have a spreading power and division power defined in vitro by specific surface measurements made by gas adsorption. For example, attapulgite (specific area of attapulgite 140 m2/g, bedeillitic 80 m2/g). For smectites, the binding capacity is very important, according to the charge (negative of sheets, positive between sheets) and the important specific surface of 100 m2 per gram. These interactions cantake place in three different sites:• in sheets for single ions, between sheets for flat or small molecules,• onthe periphery for macromolecules. The outer surface (SE) or surface covering, which defines the covering power, must be distinguished from the inner surface (SI).Kaolinite: SE= 10 to 30 m2, SI = 0 Illite and montmorillonite: 80 m2, SI: 800 m2 [3]Our topic of study is entitled “Analysis of specific surfaces of composite material: Clays of Madagascar and TiO2”. In the first part, we will carry out a bibliographical study concerning clay and specific surface and the technical analysis (Brunauer-Elmett- Teller; BET) used to determine the surface area and the porosity of the materials In the second part booked with the various results obtained with different precursors in particular clays (raw materials), the clay treated at 150°C and the composite material treated at 600°C For that, we used two types of clay, green clay and white clay. The third and the fourth part relates respectively the methodology and the characterization and the discussion of the results got compared to the bibliographical data. And finally, we will finish the article by a conclusion and the prospect for research around the polluting agent.
|
2. Bibliographical Study
- A. Clay [6,7,8]- Definition [9]It is a material of elementary layers (phyllosilicates) which pile up, introductions, disaggregate according to the condition of surrounding environment. This induced arrangement of the internal reactive surfaces, different from external surfaces, and spaces confines which are true nanoreactors. The small size of their layers of which the thickness is on nanometric scale (millionth mm) confers on clays mineral (aggregation layers in particles, then in aggregates on wide scales) all the remarkable properties in many applications that one daily mixes with since millennia. B. Surface treatmentThe term surface treatment refers to all treatments performed on the surface of a substrate, usually in the order of micron or nanometer, which gives the product surface properties different from those present in the mass.C. Specific surface [10,11,12]Measuring specific surface by nitrogen adsorption is the most common method used for material characterization. Brunauer, Emmet and Teller (BET) have designed a model of adsorption in multilayer nitrogen molecules. Surfaces measured by nitrogen adsorption correspond to external surfaces because nitrogen has no access to interfoliar spaces. The nitrogen accessible surface will depend on the texture and particle size of the materials. Specific surface gives the characterization of the solids and the porosity of the materials.
3. Methodology and Characterization
- A. Synthesis of TiO2-claysClays are activated with sulfuric acid at 80°C and washed with demineralized water to eliminate acid in excess. Then, the clays are treated at 150°C for 8 hours and are used to synthesize TiO2-bridged clays. TiO2 was synthesized in situ using the hydrolytic sol-gel process. Treated clay at 150°C is placed in a 100 mL schlenk, then isopropanol is added. The precursor of TiO2 is prepared with ALDRICH Ti(OiPr)4 and isopropanol. The mixture is then put into the schlenkunder agitation for 10 minutes and desionized water is dripped off. The mixture is heated at the 80°C under agitation for 18 hours. The gel is dried in the oven at 60°C for 2 days and then treated at 600°C under air for 1 hour.B. Characterization
 | Photo 1. Laboratory of “Chimie Moleculaire et Organisation du Solide” at the University of Montpellier II |
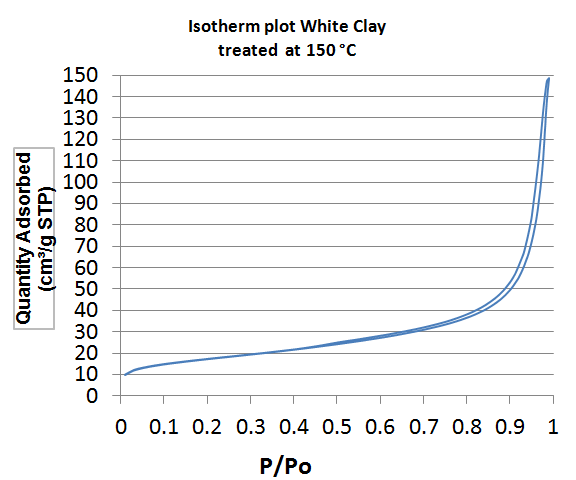 | Figure 1. Isotherm plot of White Clays treated at 150°C |
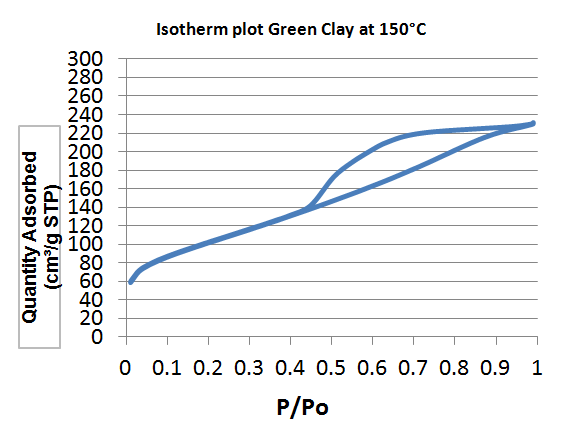 | Figure 2. Isotherm plot of Green Clays treated at 150°C |
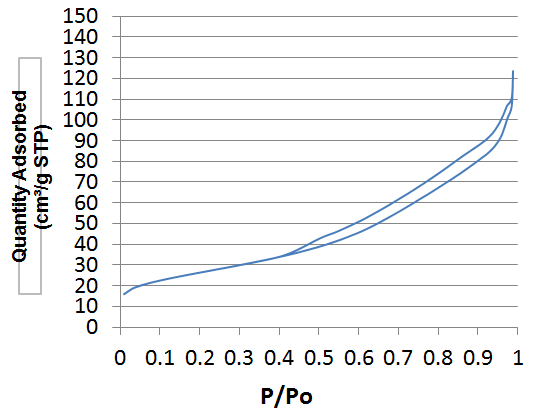 | Figure 3. Isotherm plot of White Clay with TiO2 treated at 600°C |
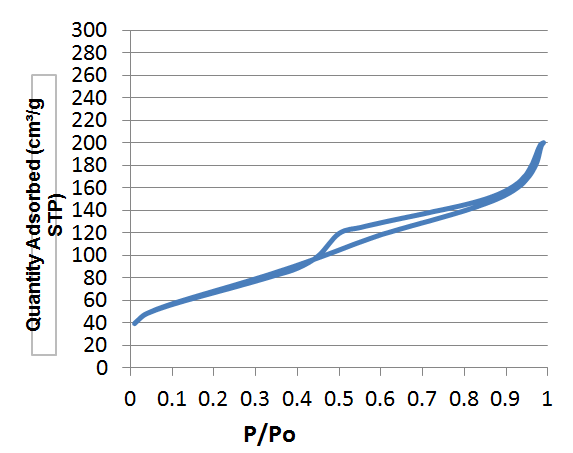 | Figure 4. Isotherm plot of Green Clay with TiO2 treated at 600°C |
4. Discussion
- By definition, the specific area (SS) also known as "Mass Area" represents the total area (AS) per unit mass (M) and is usually expressed in m2/g [13].The properties of clays are mainly controlled by their internal and external surfaces. The total surface includes the outer surface, between the clay particles, and the inner surface, corresponding to the space [14] between the sheet.White clay is also called Kaolin. It seems to be a good adsorbent. It is a clay of the Smectite family. The BET curve is the type III according to the IUPAC [15] classification. Green clay belongs to the family of the illite. Its quality is lower than those of smectite. BET analysis of clays treated at 150°C show that the specific surfaces are 60.71 m2/g for white clay (micropore surface 9.63 m2/g and outer surface 51.08 m2/g with pore size of 151.46 Å) and 244 Å, respectively. 9.52 m2/g for green clay (micropore surface: 20.51 m2/g and outer surface: 229.01 m2/g with a pore size of 49.96 Å). The size of micropores is less than 17 Å. When TiO2-clays are treated at 600°C, the specific area of white clays increases, it becomes 94.07 m2/g while pore size decreases (81.39 Å). For green clay; the specific area and the pore size are respectively about the same (247.61 m2/g) and (49.97Å). This increase in surface area in the case of white clay can be attributed to the formation of TiO2 nanocomposite on the outer surface [16] by analogy with study carried out by Luckam et al. in the case of graphènes. In the case of green clay, no change is observed as the particle size is close to that of TiO2. The grafting of TiO2 on clays surface has two advantages, at first the surface of clays has been altered and on the other hand the pore size has been reduced in the case of white clay and therefore the composite material is much more selective. For green clay, there was no change because the pore diameter is already quite small. It can be used as a filtration membrane.
5. Conclusions
- Clay is a very abundant raw material in Madagascar. There are several types of clay dependingon their color. Our study focuses on the modification of white and green clays by grafting TiO2 onto the outer surface and analyzes of specific surfaces of raw and modified clays. Raw white clay has a small specific surface area compared to green clay. When TiO2 is fixed to the surface, there is a marked decrease in pore size and an increase in the surface for white clay, while for green clay there is no change since pore size has not changed, TiO2 is fixed to nanometric clay particles. The active surface area and pore structure of catalysts influence production rates. Limiting the pore size allows only molecules of desired sizes to enter and exit, creating a selective catalyst that will produce primarily the desired product.I would like to thank the laboratory team of the Molecular Chemistry and Solid Organization (MCSO) Unit of the University of Montpellier II France for the analysis of the samples.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML