-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2019; 9(2): 29-33
doi:10.5923/j.ijmc.20190902.01

Preparation of Carboxymethyl Chitosan Bombyx Mori Nanofibers by Electrospinning Process
Sattarova Dilfuza Maksudovna
Namangan State University, Namangan, Uzbekistan
Correspondence to: Sattarova Dilfuza Maksudovna, Namangan State University, Namangan, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study, the electrospinning of carboxymethyl chitosan isolated from silkworm Bombyx mori has been investigated. For this purpose, samples of carboxymethylchitosan were synthesized, and their molecular mass characteristics were also studied. Distilled water, acetic and trifluoroacetic acids were used as solvents in the preparation of polymer solutions. The nanofibrous structure based on carboxymethylchitosan in the solvent trifluoroacetic acid with an average diameter of nanofibers of 156–282 nm was obtained by varying the conditions of the electrospinning process.
Keywords: Chitosan, Carboxymethyl chitosan, Nanofibers, Electrospinning
Cite this paper: Sattarova Dilfuza Maksudovna, Preparation of Carboxymethyl Chitosan Bombyx Mori Nanofibers by Electrospinning Process, International Journal of Materials and Chemistry, Vol. 9 No. 2, 2019, pp. 29-33. doi: 10.5923/j.ijmc.20190902.01.
Article Outline
1. Introduction
- One of the promising areas of the field of nanotechnology is the method of electrospinning of polymer solutions for preparing nanofibers with ultra-developed structure and porosity. Nonwoven fibrous materials obtained by electrospinning of polymer solutions are widely used in different spheres.Silk production is traditionally developed in Uzbekistan. Waste silkworm pupae amount to about 10-3 thousand tons per year. This source of raw materials can use as a base for the production of chitin and its derivatives for various purposes.One of the promising derivatives of chitosan (CS) is carboxymethylchitosan (CMCS). CMCS has a high moisture-adsorption and moisture-holding capacity, as well as the best biological, chelating and sorption properties in comparison with CS. CMCS has been widely studied because of its available synthesis, ampholytic character and wide range of use [1,2].CMCS is of great interest for scientific research, based on its available synthesis and ampholytic character, which determine the great potential for biomedical and technological applications such as controlled release of drugs and pH sensitive drug delivery, regeneration of the nervous system, ultrafiltration, etc. [3-6].It has been determined that low flexibility of the CMCS chain and the high charge of its molecule leads to a high repulsive force that prevents the cohesion of the chains and thus creates additional obstacles in the formation of fibers [7].Electrospinning of CMCS aqueous solutions was possible with the addition of water soluble polymers such as PEO, PAA, and PVA. Electrospun nanofibers were successful prepared from blended solution with an equal ratio of O-CMCS (89 kDa and DS = 0.36) and PVA with an average diameter of nanofibers 130 nm [8].The preparation of nanofibres from 3–9% aqueous solution of CMCS in the presence of 8% PVA aqueous solution in various mass ratios was studied. The antibacterial properties of produced CMCS / PVA nanofibers on Gram (+ ve) and Gram (-ve) bacteria were studied [9].The process of electrospinning of CS and CMCS with the aim of forming ultrathin nanofibers for medical application is being studied by many scientists.Despite a lot of research in the field of studying the electrospinning process of CMCS, there is less information about the features of the formation of CMCS nanofibers isolated from silkworm Bombyx mori.The purpose of this work is to form nanofibers from CMCS Bombyx mori by electrospinning for potential application as wound dressing.
2. Experimental
2.1. Materials and Equipment
- The materials used in this study include: Chitosan Bombyx mori which was derived from silkworm pupae (Uzbekistan), trifluoroacetic acid (TFA) from Aladdin chemistry Co.LTD, acetic acid from Beijing Chem. Works, Beijing SHIJI. All chemicals and solvents were used without further purification.Ubbehlode type viscometer for viscosmetry for estimating the molecular weight, GPC system incorporated by Waters, NMR spectrometer Bruker 600 MHz for studying the functional groups of CS, electrospinning apparatus with high voltage supply (DW-P503-1ACDFO, Tianjin Dongwen High Voltage Power Supply Limited Company, China), SEM apparatus JSM-5610 for observing the structure of fibers and calculating their mean diameters.
2.2. Chitin Isolation Process and Chitosan Production
- The isolation of chitin from Bombyx mori silkworm pupae was carried out according to work [10] with some modification.Demineralization, in which the dried pupal powder was immersed in 3% HCL solution and with constant stirring the reaction, was carried out at 100°C for 30 minutes, after which the mass was washed with distilled water several times until pH was neutral. The deproteinization process was carried out with 4% NaOH solution at 80°C for 24 hours (weight ratio 1:10). After filtration, the mass was washed several times with distilled water. Drying was carried out at 60°C for 24 hours. It is known that the composition of a dry pupa contains about 32% of fats. To eliminate fats, an extraction process was carried out on a Soxhlet apparatus with a capacity of 2000 cm3 in an acetone solvent for 3–4 hours.Bleaching was carried out with 3% H2O2 for 30 minutes.Chitin was deacetylated using 40% NaOH solution at 100°C for 8 hours (1:10) with constant stirring. The product yield was about 3.67%.
2.3. Carboxymethylchitosan Preparation
- Synthesis of CMCS from CS Bombyx mori was carried out according to work [11]. CS sample (2 gr) was thoroughly mixed with 20 ml of 50% NaOH for one hour on a magnetic stirrer. Then the resulting mixture was placed in a refrigerator at -20°C at 16 hours. After that, the mixture was set aside for complete thawing at room temperature, to which 50 ml of isopropyl alcohol was subsequently added and thoroughly mixed on a magnetic stirrer for 30 minutes, and then in a water bath at 50°C. Monochloroacetic acid (10g) in isopropanol (30 ml) was added drop wise over 30 minutes to the reaction mixture. The reaction was carried out for 12 hours at 50°C with constant stirring. The resulting liquid fraction was decanted, followed by the addition of 100 ml of methanol. The suspension was neutralized with glacial acetic acid. The mixture was filtered and washed several times with methanol. For complete purification, the resulting mass was dissolved in distilled water; the undissolved part was filtered. Carboxymethyl chitosan was precipitated with methanol, and then dried under vacuum at 60°C.
2.4. Conductometric Titration
- The degree of deacetylation (DD) of the Bombyx mori sample of the CS and the degree of substitution (DS) of the CMCS were determined by conductometric titration according to method [12]. The calculations of the DD of the CS were calculated from the results of conductometric titrationDD= [base] (V2-V1)161/mwhere [base] is the concentration of sodium hydroxide solution (mol / l-1), V2 and V1 are the volumes of consumed solution of sodium hydroxide for titration (ml), 161 molar monomer mass (С6H11О4N)and m - the chitosan mass (mg).
2.5. Viscosity
- The viscosities of chitosan samples was determined in a solvent of СH3COOH / 0.2 M NaCL and N, O-CMCS in H2O + 2% NaCl at 25°C using an Ubbelodhe type capillary viscometer. Six dilutions with the solvent were used for each sample. The flow time data were used to calculate the relative viscosity, the reduced viscosity and then the intrinsic viscosity.The molecular mass of the polymer was calculated by the Mark-Kun-Houwink equation
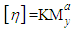 Where [η] is the intrinsic viscosity, Mv is the average viscosity molecular weight, the values of K = 1.81x 10-5, a = 0.93 respectively for this system for CS [13] and for CMCS the values of К = 7,92*10-5, α = 1.00 [14].
Where [η] is the intrinsic viscosity, Mv is the average viscosity molecular weight, the values of K = 1.81x 10-5, a = 0.93 respectively for this system for CS [13] and for CMCS the values of К = 7,92*10-5, α = 1.00 [14].2.6. Gel Permeation Chromatography
- The molecular weight and polydispersity index of the samples were determined using GPC on a Waters liquid chromatograph. The flow rate was set at 0.50 ml / min under psi-612 pressure. The chromatograph system incorporated a Waters 1515 HPLS series pump. The system also included Waters 2414 refractive index detector. The column used was WATO11545 provided by Waters with dimension of 300mm x 7.8 mm. The temperature of the column was maintained at 35°C.Polyethylene glycol was used as a standard for calibration. The solvent used was 0.5 M CH3COONa / 0.5 M CH3COOH.
2.7. 1H NMR Spectroscopy
- 5 mg of CS and N, O- CMCS were placed in 5 mm NMR spectrometer tubes containing 0.5 ml of a solution of 2% DCL in D2O and 0.5 ml of D2O, and were set aside for 3 hours at 25°C for complete dissolution. The 1H NMR spectrum was obtained with a spectrometer at 600 MHz and 25°C. The 1H NMR spectrum of the samples showed all peaks corresponding to the chitosan and carboxymethyl chitosan structures.The 1H NMR spectrum of CS Bombyx mori sample in Fig.1(a) shows the distribution of protons in the following order exhibited a singlet at 1.91 ppm characteristic of methyl hydrogens of GlcAC units, a signal at 3.01ppm related to the hydrogen bonded to C (2) of GlcN units, the set of signals 3.7-3.9 ppm corresponding to the hydrogens H(3)-H(6) from GlcN unit and the hydrogen bonded to C (2) of GlcNAc unit, 4.71 ppm are atributed to the anomeric carbon (C(1) of GlcAc and GlcN(H(1)) units.A comparative analysis of the 1H NMR spectrum of the carboxymethyl chitosan Fig.1(b) show all the characteristic peaks present in the spectrum of chitosan, and new peaks at 3.24 and 4.24 ppm corresponding to the two protons of the carboxyl groups of N-CH2 and O-CH2- protons in positions 2 and 6 were detected. This indicates the formation of N,O-CMCS Bombyx mori.
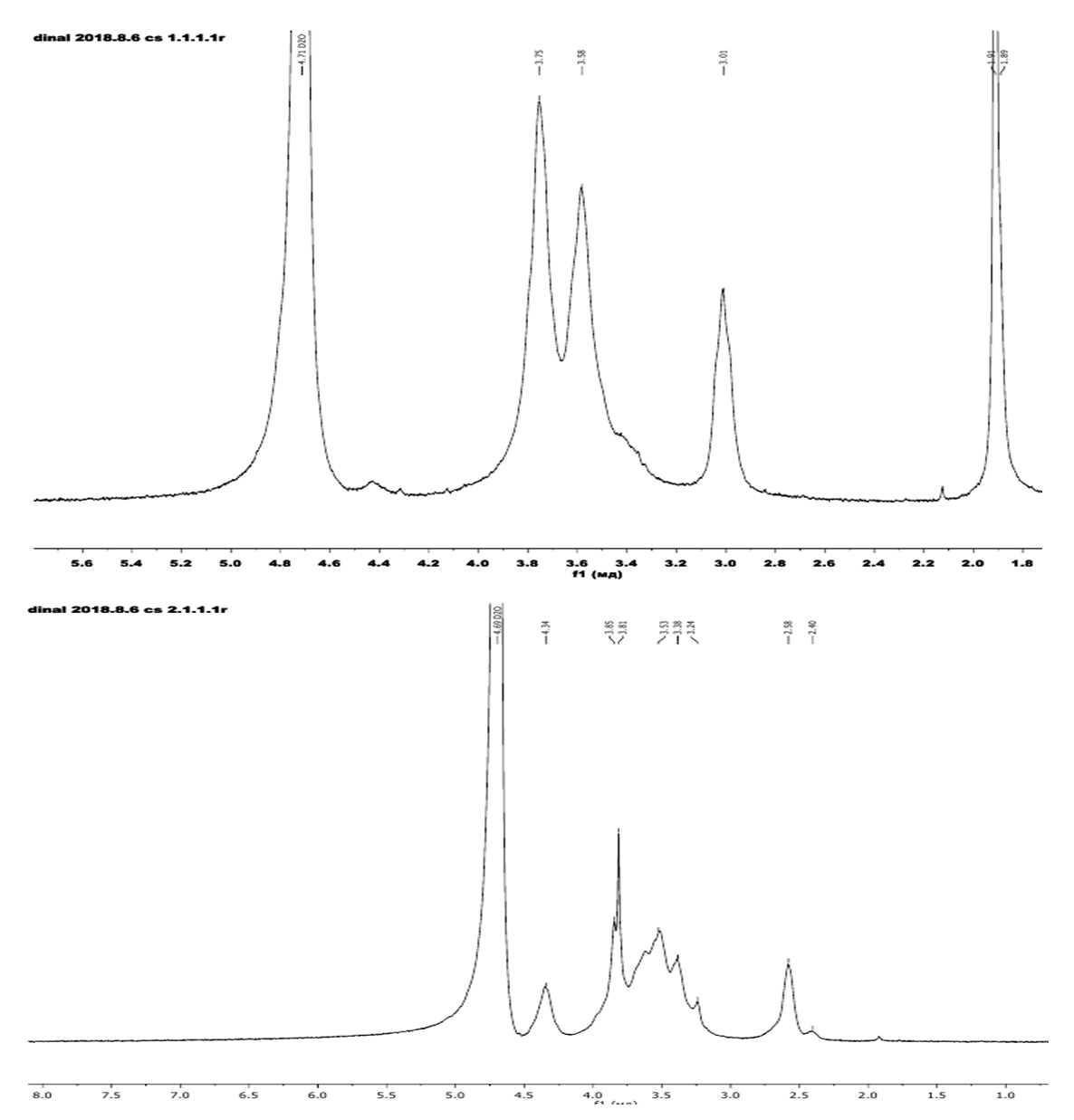 | Figure 1. 1H NMR spectrum of chitosan Bombyx mori (a) and N, O-CMCS (b) |
2.8. Electrospinning Process
- For the electrospinning of CMCS, the polymer solution was loaded in a 5 ml syringe with blunt-end stainless steel needle (inner diameter-0.6 mm). The needle was connected to a high voltage supply. An aluminum foil was used as collector connecting to the ground of the power supply and was placed perpendicular to the needle. The applied voltage was (25-40kV) and distance between the needle tip and the collector was 20 cm. Electrospinning was carried out at ambient temperature with the humidity of 40-50%. The resulting CS nanofibers were dried at room temperature for 24 h.
3. Result and Discussion
- According to the results of 1H NMR spectroscopy of the samples, all peaks corresponding to the structures of chitosan and carboxymethyl chitosan were detected.First the distilled water than concentrated acetic acid (70-90%) were tried as some solvents for CMCS but the formation stable jet was not observed which is necessary for electrospinning process. TFA was chosen as a suitable solvent for an efficient electrospinning process because of the ability to form salts with amino groups of CS, which cause repulsive forces and leads to the problematic formation of a solution of pure CS.Several solutions with different concentrations were prepared for the selection of the optimal viscosity.Attempts to form nanofibers from solution with lower concentrations led to the formation of nanofibers with a high content of droplets at 40 kV in Fig.2.(a) and (b).In the case of a 7% solution of CMCS, the formation of stable jet and the collecting of uniform nanofibers on the electrode were observed. Fig.2.(c) shows that under these conditions the electrospinning process produced continuous CMCS nanofibers with less formation of beads.
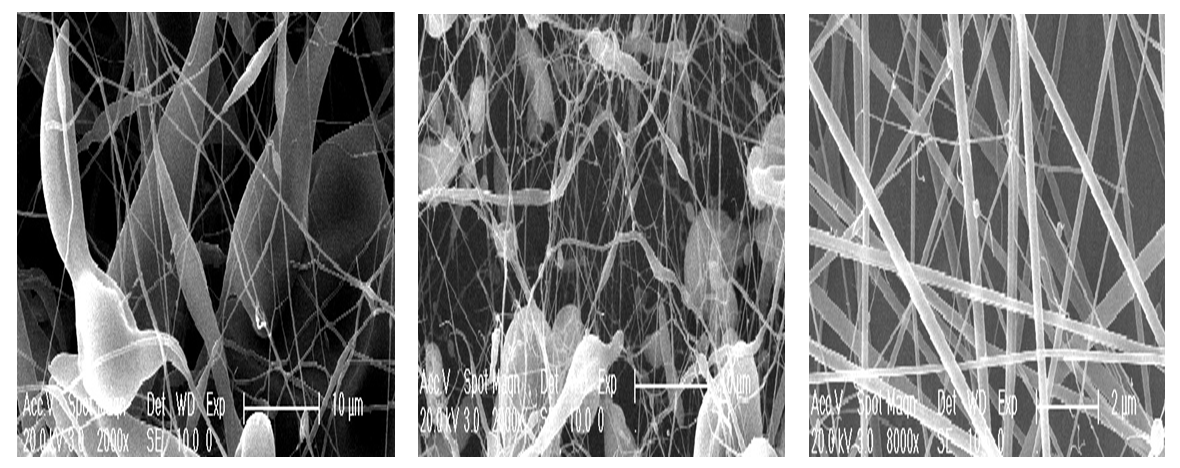 | Figure 2. SEM images of 5 wt %CMCS (103kDa and with DS 0.6) in TFA (a), 6 wt % CS in TFA (b)and 7 wt %CS in TFA (c) tip needle –collector distance 15 cm at 40 kV |
4. Conclusions
- The electrospinning of carboxymethyl chitosan isolated from silkworm Bombyx mori has been investigated. Distilled water and acetic acid (70-90%) were tried as solvents for CMCS but the formation stable jet was not observed. TFA allowed the formation of stable jet and was found a suitable solvent for an efficient electrospinning process. The nanofibrous structure based on carboxymethylchitosan in the solvent trifluoroacetic acid with an average fiber diameter of 156-282 nm was obtained from a 7% CMCS solution with applied electrical voltage of 40 kV by varying the conditions of the electrospinning process. From all the concentrations of CMCS samples, a solution with a molecular weight of CS (103kDa and with DS 0.6) was effective in forming smooth, better-quality fibers compared with lower molecular weight (78kDa and DS 0.65) samples.The regularity of the decrease in the mean diameter of nanofibers with increasing voltage was determined.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML