-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2019; 9(1): 23-27
doi:10.5923/j.ijmc.20190901.03

Synthesis of Methylacrylate and Acrylic Acid Copolymers and Their Application as Materials for Restoration
N. I. Bozorov, V. O. Kudyshkin, S. Sh. Rashidova
Institute of Polymer Chemistry and Physics Academy of science of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: N. I. Bozorov, Institute of Polymer Chemistry and Physics Academy of science of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The radical mechanism was used to synthesize copolymers of methylacrylate and acrylic acid in the bulk and dioxane. By regulating the composition of the copolymer, macromolecules with both hydrophilic and hydrophobic properties were obtained. It was shown that the obtained copolymers satisfy the basic criteria required for the application in the practice of restoration of archaeological objects of different nature.
Keywords: Methylacrylate, Acrylic acid, Radical copolymerization, Copolymer composition solubility, Archeological object, Model
Cite this paper: N. I. Bozorov, V. O. Kudyshkin, S. Sh. Rashidova, Synthesis of Methylacrylate and Acrylic Acid Copolymers and Their Application as Materials for Restoration, International Journal of Materials and Chemistry, Vol. 9 No. 1, 2019, pp. 23-27. doi: 10.5923/j.ijmc.20190901.03.
Article Outline
1. Introduction
- Copolymers of methylacrylate (MA) and acrylic acid (AA) have been known for a long time [1, 2]. However, there is a little information about the properties of these copolymers and the ways of their practical application. By regulating the synthesis conditions, copolymers can be synthesized in a fairly wide range of compositions. The combination in the macromolecular chain of monomer links containing the group - COOH and - O-CH3 makes it possible to obtain materials in a wide range of hydrophilic-hydrophobic properties. This opens interesting ways of practical application of this copolymer in the practice of restoration and conservation of archaeological objects. It is known, that polybutylmethacrylate, in particular, is widely used for these purposes [3, 4]. Copolymers of acrylic monomers such as butylacrylate and methylacrylate to exhibit adhesive properties to various surfaces (stainless steel, polyethylene, glass and Si-wafer), what makes it possible to use them as pressure sensitive adhesives [5]. Copolymerizations of unsaturated compounds with different ratios of methacrylic monomers (Methylmethacrylate, Butylmethacrylate) exhibit good adhesion, flexibility and waterproofing performance may be used in the paint industry [6]. Different copolymers of hydroxyethyl acrylate and methyl methacrylate were synthesized by solution polymerization using azo-bis-(isobutyronitrile) (AIBN) as initiator and dimethylformamide as a solvent. The copolymers exhibited good thermal stability, flexibility, and adherence [7]. There is also information that the copolymers MA:AK successfully tested in the practice of paper restoration [8, 9].In this paper has shown the possibility of synthesis of statistical copolymers MA and AA of different compositions and shows the perspectives of their practical application for archaeological objects restoration.
2. Experimental
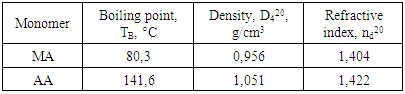
- During of investigations used industrial monomer MA produced “NAVOIYAZOT” JSC (Uzbekistan) and ice AA produced HIMEDIA Laboratories (India). The monomers were rectified by vacuum distillation. The distilled monomers had the following characteristics:
 Copolymers were synthesized in a flask placed in a water bath in a nitrogen atmosphere, in the presence of azobis (isobutyronitrile) (AIBN) as initiator at 60°C. Reprecipitation of the polymers was performed with hexane. The composition of the copolymer was determined by elemental analysis by titration of carboxylic groups.IR spectra have been recorded using Specord 75 IR spectrometer. The samples were in the form of films or tablets with KBr.Intrinsic viscosities of polymer solution were measured by using Ubbelohde viscosimeter [10].
Copolymers were synthesized in a flask placed in a water bath in a nitrogen atmosphere, in the presence of azobis (isobutyronitrile) (AIBN) as initiator at 60°C. Reprecipitation of the polymers was performed with hexane. The composition of the copolymer was determined by elemental analysis by titration of carboxylic groups.IR spectra have been recorded using Specord 75 IR spectrometer. The samples were in the form of films or tablets with KBr.Intrinsic viscosities of polymer solution were measured by using Ubbelohde viscosimeter [10]. 3. Results and Discussion
3.1. Synthesis of Copolymers
- Both AA and MA relates to the active monomers, because they have in their composition a carbonyl group directly associated with a double bond. In the structure of monomers, π–π conjugation is realized, which increases the reactivity of the double bond. Both monomers have a positive polarity factor e, which indicates a positive charge on the β-carbon double bond atom.
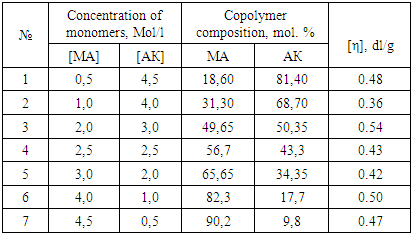
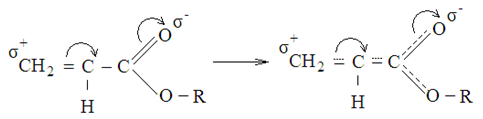 were R: – Н or СН3Synthesis of the copolymers of MA and AA in the solution of dioxane and in the bulk was carried out. Figure 1 shows the dependence of the copolymer composition on the molar ratio of the monomers in the initial reaction mixture.
were R: – Н or СН3Synthesis of the copolymers of MA and AA in the solution of dioxane and in the bulk was carried out. Figure 1 shows the dependence of the copolymer composition on the molar ratio of the monomers in the initial reaction mixture.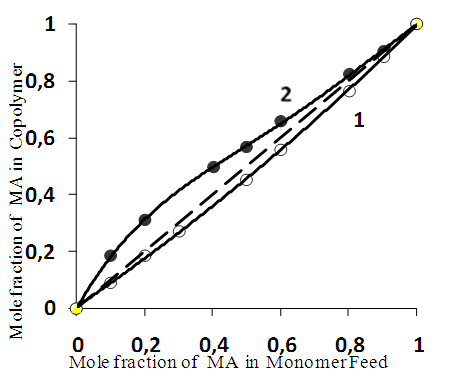 | Figure 1. Schematic Representation of the Copolymers composition vs. Monomer Feed in dioxane (1) in bulk (2). [AIBN]= 6.8∗10-3, Т= 60°C. Yield< 10% |
 The difference in the copolymerization constants of AA in the bulk and in dioxane could be explained as follows. It is known that AA in the bulk there is a dimer of the following structure [2]:
The difference in the copolymerization constants of AA in the bulk and in dioxane could be explained as follows. It is known that AA in the bulk there is a dimer of the following structure [2]: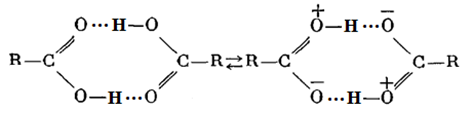 According literature it is known that π - electrons of the carbonyl group closing the conjugation system can participate in the hydrogen bond, and π - electron cloud can be transferred through the hydrogen bond. In dimeric ring formed is common to both molecules of conjugation circuit. Therefore, in the dimers of AA, a double bond in a greater degree associated with π - electronic system of the substituent and an unpaired electron in the corresponding radical is largely delocalized than in the “isolated” AA molecules. This is confirmed by the fact, that the Q-factor (resonance stabilization factor) characterizing the degree of conjugation of the double bond with the substituent in AA dimers is greater than in the complexes of the same acid with the solvent. Indeed, according to [2], the value of resonance stabilization factor for AA in bulk QAA=0.7, in the solution QAA =0.15. These data indicate that during polymerization in the solvent, the AA dimer is destroyed, which leads to decrease of the resonance stabilization factor and, accordingly, to decrease of the AA copolymerization constant. In the copolymerization in dioxane r1< 1 and r2< 1, so the dependence (Fig. 1) crosses the azeotrope line at the molar ratio MA:AA ~ 95:5. From the dependencies shown in Fig. 1 it follows an important conclusion. For almost any composition of the monomer mixture, can be chosen a solvent concentration at which the composition of the copolymer will coincide with the composition of the monomer mixture. In this case, the composition of the monomer mixture don't change with increasing conversion, which contributes to the production of copolymers with high homogeneity in composition, since the conversion component of the composite heterogeneity is excluded. The synthesized copolymers were also characterized by IR spectroscopy (Figure 2).
According literature it is known that π - electrons of the carbonyl group closing the conjugation system can participate in the hydrogen bond, and π - electron cloud can be transferred through the hydrogen bond. In dimeric ring formed is common to both molecules of conjugation circuit. Therefore, in the dimers of AA, a double bond in a greater degree associated with π - electronic system of the substituent and an unpaired electron in the corresponding radical is largely delocalized than in the “isolated” AA molecules. This is confirmed by the fact, that the Q-factor (resonance stabilization factor) characterizing the degree of conjugation of the double bond with the substituent in AA dimers is greater than in the complexes of the same acid with the solvent. Indeed, according to [2], the value of resonance stabilization factor for AA in bulk QAA=0.7, in the solution QAA =0.15. These data indicate that during polymerization in the solvent, the AA dimer is destroyed, which leads to decrease of the resonance stabilization factor and, accordingly, to decrease of the AA copolymerization constant. In the copolymerization in dioxane r1< 1 and r2< 1, so the dependence (Fig. 1) crosses the azeotrope line at the molar ratio MA:AA ~ 95:5. From the dependencies shown in Fig. 1 it follows an important conclusion. For almost any composition of the monomer mixture, can be chosen a solvent concentration at which the composition of the copolymer will coincide with the composition of the monomer mixture. In this case, the composition of the monomer mixture don't change with increasing conversion, which contributes to the production of copolymers with high homogeneity in composition, since the conversion component of the composite heterogeneity is excluded. The synthesized copolymers were also characterized by IR spectroscopy (Figure 2).  | Figure 2. IR - spectra of poly-MA(1), poly-AA (2), MA:AA copolymer (3) |
3.2. Copolymers Solubility
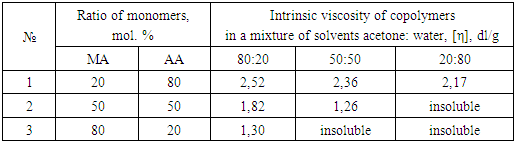
- MA: AA copolymers have different solubility depending on their composition. Samples containing more than 50% AA at room temperature do not dissolve in DMFA, but are well soluble in water, ethanol and dioxane. Samples which contain in their composition 50% or less of AA, easily soluble in dioxane and DMFA. Thus, copolymers, depending on their composition, have different affinity to solvents. We also investigated the influence of the composition of the copolymer in their intrinsic viscosity ([η]) in a mixture of solvents acetone: water (Table 1).
|
|
3.3. Experiments on Models of Archaeological Objects
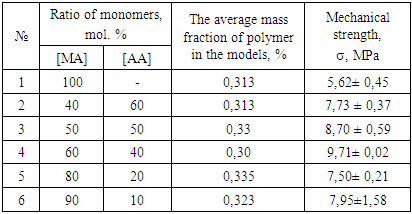
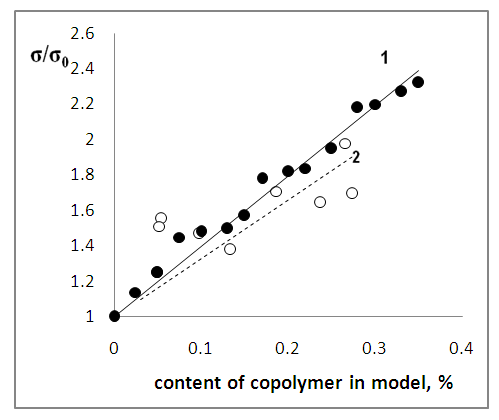
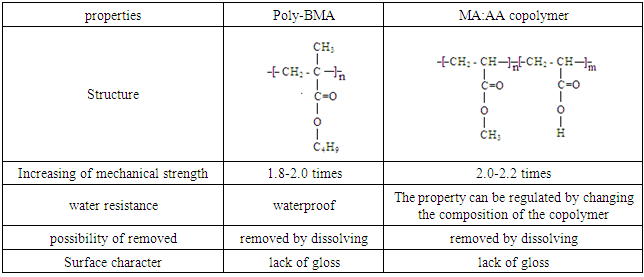
- Polymer materials used for the restoration of archaeological objects should satisfy a number of criteria. First of all, it should provide an increase in the strength of objects. Also, it should not change the appearance of the object. The surface after treatment should not look like lacquered. It is also important that the polymer retains its linear structure for a long time so that it can be removed from the object by dissolving. The obtained MA:AA copolymers were tested on models of archaeological objects. The models are samples in the form of cubes made of a mixture of sand and clay in the ratio sand:clay = 3:7 by pressing and then drying at room temperature to a constant mass. Copolymers were dissolved in acetone. Concentration of solutions up to 5%. The copolymer solutions were then applied with a brush to the models, dried to a constant mass, and then tested for mechanical strength. The experiments were carried out on the ZWICK testing machine, adapted to the press.Table 3 shows the dependence of the mechanical strength of models (σ) on the composition of the copolymer by which they were impregnated. The average polymer mass applied to the model was 0.090 ±0.011 g.
|
|
4. Conclusions
- Thus, in the conditions of radical initiation the copolymers MA and AA of different composition were synthesized. It is established that the copolymer MA: AA has all the basic criteria necessary for its application in the practice of restoration of archaeological objects. Impregnation of models with copolymer solutions leads to an increase in their mechanical strength. In this case, the copolymer retains the linear structure and can be removed from the object if necessary.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML