Amra Bratovcic1, Sanela Nazdrajic2, Amra Odobasic1, Indira Sestan1
1Department of Physical Chemistry and Electrochemistry, Faculty of Technology, University of Tuzla, Tuzla, Bosnia and Herzegovina
2Faculty of Education, University “Dzemal Bijedic” in Mostar, Mostar, Bosnia and Herzegovina
Correspondence to: Amra Bratovcic, Department of Physical Chemistry and Electrochemistry, Faculty of Technology, University of Tuzla, Tuzla, Bosnia and Herzegovina.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
In this paper, two different groups of liquid soaps were prepared. The first group of samples consisted of anionic surfactant (SLES), amphoteric surfactant (BETAIN) and nonionic surfactant (DEA). The second set of samples consisted of anionic surfactant and two nonionic surfactants. The aim of this work is to investigate the influence of the type of surfactant as well as the mass fraction of surfactants on the physicochemical properties of liquid soap. The surface tension, electrical conductivity and density for different concentrations of all examined type of surfactants have been determined as well as the critical micelle concentration (CMC). Moreover, the studies have shown that by increasing concentrations of zwitterionic (amphoteric) surfactant, and by decreasing concentration of nonionic surfactant, a mild decrease in pH value and viscosity increase occurred. In contrast, with increasing polyglycoside concentrations and decreasing concentration of DEA, a mild increase in pH but a decrease in viscosity was observed. In order to monitor the stability of the liquid soaps obtained, the appearance, color and odor were observed at three different temperatures at +4°C, room temperature and at + 40°C, in the dark and under UV light during the three months.
Keywords:
Liquid soap, Surfactants, pH, Viscosity, Surface tension, Electrical conductivity, Critical micelle concentration, Stability
Cite this paper: Amra Bratovcic, Sanela Nazdrajic, Amra Odobasic, Indira Sestan, The Influence of Type of Surfactant on Physicochemical Properties of Liquid Soap, International Journal of Materials and Chemistry, Vol. 8 No. 2, 2018, pp. 31-37. doi: 10.5923/j.ijmc.20180802.02.
1. Introduction
There is always a need for a good and quality handwashing agent, especially for health reasons that accompany a person who is exposed to various impurities and microorganisms. For this reason, we face the challenge of making liquid soap that will meet the needs of our customers by their quality, color, fragrance, appearance and stability. Soaps are agents for washing based on synthetic surface active agents (surfactants) and auxiliary components. A surfactant molecule is composed of a hydrophilic head and a hydrophobic tail [1]. Generally, based on the nature and the type of the surface active moiety group present in the molecule, surfactants are classified as anionic, cationic or non-ionic surfactants and in case both cationic and anionic centers are present in the same molecules, they are termed as zwitterionic (amphoteric) surfactants [2]. Soap is chemically defined as the sodium or potassium (alkali) salts of fatty acids or similar products formed by the saponification or neutralization, by which triglycerides (fats and oils) or fatty acids are transformed with organic or inorganic bases into the corresponding alkali salt mixtures of fatty acids.
1.1. The Basic Ingredients for Liquid Soaps
The basic ingredients for liquid soaps are surfactants, particularly of the anionic type (sodium laureate sulfate, sodium lauryl sulfate and other sulfates). Liquid detergents are typically formulated with sodium salts and surfactants; the main reason being their low price. In addition, compounds of this type have a high capacity to increase the viscosity of detergents [3].Surfactants are widely used and many topical pharmaceutical formulations, cosmetics, antiseptics, shampoos, detergents, creams and lotions contain surfactants. Surface active products are for to their amphiphilic properties used as emulsifiers, suspending, wetting, solubilizing and stabilizing agents [4].
1.2. Classification of Surfactants
The word surfactant is an abbreviation for surface active agent. A surfactant is defined as a compound that can reduce the interfacial tension between two immiscible phases. [5]. Surfactants are a special class of versatile amphiphilic compounds that possess spatially distinct polar (hydrophilic head) and non-polar (hydrophobic tail) group [6].Surfactants are among the most essential and important ingredients encountered in laundry detergents, dishwashing detergents, liquid soaps, cleaning products, cosmetic hair care and personal care products which represent the main applications of these compounds. The detergents and personal care products use nearly 60% of all surfactants. Their function is to remove soil (oil, grease, dust, particles...) from solid surfaces and to keep it in suspension in the wash solution, preventing redeposition on clothes. The detergency is essentially governed by two factors: the solubility of the surfactants and their critical micelle concentration [7].A surfactant is characterized by its tendency to adsorb at surfaces and interfaces. They show interesting phenomena in solution by modifying the interfacial and bulk-solvent properties. 1.2.1. Anionic surfactants are often accompanied by the small positive ions such as sodium or ammonium to balance the negative charge. The anionic surfactants carry a negative charge in water. Anionic surfactants are used in greater volume than any other surfactant class and are used in most detergent formulations. One reason is the ease and low cost of manufacture. Anionic surfactants mostly contain carboxylates, sulfonates, sulfates or phosphates moiety as hydrophilic head group. Many alkyl sulphates are used as detergents, but by far the most popular member of this group is sodium lauryl sulphate which is compatible with dilute acid and with calcium and magnesium ions. The lower-chain-length compounds, around C12, have better wetting ability, whereas the higher members (C16–C20) have better detergent properties [6].1.2.2. Nonionic surfactants are the second largest surfactant class and have either polyether or polyhydroxyl as polar group to increase water solubility. Nonionic surfactants do not have any charge on them. They do not ionize. They are usually mild and usually used as emulsifiers, conditioning ingredients, solubilizing agents, foam stabilizers etc. Nonionic surfactants have long been recognized as compounds with a low irritating effect, and hence are widely used in topical products [8].1.2.3. Zwitterionic (amphoteric) surfactants contain both cationic and anionic centers, the ionic behavior of which is altered according to pH of the solvent. These surfactants are effectively used in personal care and household cleaning products because of the excellent dermatological properties of the surfactants. Zwitterionic surfactants are used a lot in shampoo formulations as secondary surfactants because they do not have good cleansing properties and don’t function well as emulsifiers. They help boost foam, improve conditioning and even reduce irritation. They are also used for baby shampoos and other cleansing products that require mildness. Examples include sodium lauriminodipropionate, disodium lauroamphodiacetate, or cocamido propyl betaine (most popular). An important property of the zwitterionic surfactant is in the ionization state of their molecules, which dependent on the pH of the solution. The ionization state of the surfactant molecules in the bulk of solution could be markedly different from the ionization state of the same molecule when it is incorporated in to an adsorption monolayer. They are applicable in a wide pH range and have en excellent biodegradability [9].1.2.4. Cationic surfactantsMany long-chain cations, such as amine salts and quaternary ammonium salts, are used as cationic surfactants when dissolved in water. Those containing carboxylate ions are known as natural soaps. However, their use is generally limited to that of antimicrobial preservatives because of their bactericidal activity [10].
1.3. Micelle and Critical Micelle Concentration
The concept of micelles in solution was developed by James William Mc Bain and coworkers at the University ofBristol in Bristol, England in the early twentieth century [11]. Micelles are aggregates formed by surfactants above their critical micelle concentration (CMC) and are composed of a hydrophilic surface and hydrophobic core. This specific structure makes the micelles capable of establishing chemical and physical interactions with either hydrophilic or lipophilic substances [12, 13].One of the most interesting properties of surfactants in solution is their ability to self-aggregate to form association colloids known as micelles, accompanied by an overall decrease in the free energy of the system [14]. When the concentration of the surfactant molecules in the bulk of the solution exceeds a limiting value, the surfactant molecules self-aggregate to form micelle which is manifested by an abrupt change in many physicochemical properties [15].The narrow concentration range over which these changes occur is known as critical micelle concentration (CMC) and is perhaps the most important characteristic property of a surfactant [16]. The tendency of a surfactant to form micelle in solution is largely dependent on the type and nature of the surfactant. Surfactants with longer hydrophobic tail (i.e. more hydrophobicity) generally exhibit greater tendency towards micelles formation. With increase in the length of the hydrophobic tail, the hydrophobic effect becomes stronger and consequently the CMC decreases and larger micelles are formed.
1.4. The Main Objective
The main objective of present research is to determine the viscosity of several surfactant solutions and to investigate the influence of the type of surfactant as well as the mass fraction of surfactants on the viscosity. The surface tension, electrical conductivity and density for different concentrations of different type of surfactants have been determined as well as the critical micelle concentration.
2. Experimental Part
2.1. Materials and Methods
Beaker 1000 mL, stirrer, hot plate, balance, pipet, graduated cylinder 250 mL, thermometer, pH meter, viscometer.- Determination of physical properties (surface tension, viscosity, electrical conductivity) of different concentration concentrations- Determination of Critical Micelle Concentration (CMC)- Tensiometer for surface tension determination using digital tensiometer Digital - Tensiometer K10T Krüss- Viscosimetric method using two viscometer Brookfield DV-I and Viscometar Model: RVDVI +- Method for Determination of Electrical Conductivity by pH/Conductometer Mettler Toledo MPC 227 and Conductometric Electrode Mettler Toledo InLab 730 NTC, 0 ... 1000 mS, 0 ... 100°C- Sensory analysis to determine color, smell and appearance
2.2. Chemicals
An aqueous solution of Sodium lauryl ether sulfate (SLES) Genapol LRO, Clariant Switzerland, an anionic surfactant commonly used in industry, cocoamidopropyl betaine (BETAIN), DEA (Amidet B112, Vrbeks, Serbia), polyglycoside (Glucopon 650 EC, BASF, Germany), glycerin, preservative, dye, fragrance, citric acid, sodium chloride, water.Genapol LRO liquid is a sodium lauryl ether sulfate (SLES) which is a strong foaming agent and unsusceptible to water hardness. It is also a wetting agent.Cocoamidopropyl betaine is an amphoteric synthetic detergent that has been increasingly used in cosmetic and personal hygiene products (shampoo, contact lens solutions, skin care products, cleanser, liquid soaps, antiseptics, and gynecologic and anal hygiene products) because it induces relatively mild skin irritation [17].DEA (amidet B112) is a liquid non-ionic surfactant with good thickening and foaming properties. It is soluble in anionic surfactant mixtures. It also performs as re-fatting agent on the skin, controlling strong degreasing effect of the anionic surfactant on the skin. It acts also as foam booster, improving quality of the foam.Polyglycoside (Glucopon 650 EC) provides excellent dishwashing performance especially in combination with anionic and amphoteric surfactants. In combination with anionic surfactants the viscosity can be improved by electrolyte addition. This product is well suited for the manufacture of manual dishwashing agents. It exhibit very low dermal irritation and is classified as “non irritating to skin”.
2.3. Synthesis of the Liquid Soap
SLES has been dissolved in 300 g of warm water and mixed it 20 min. Then, surfactants were added and mixed it about 10 minutes until clear and homogeneous solution appeared. Subsequently, glycerin was added. Afterwards, it was noticed turbidity of solution. Afterwards, the solution of citric acid has been added in order to adjust pH. Stirring is continued. The preservatives, dye and fragrance were added and stirred well until homogeneous and clear solution. Then the salt, sodium chloride was added slowly and viscosity was controlled. When the viscosity was no more changed, the stirring process was stopped.Katarzyna Staszak and coworkers showed that the effect of added sodium chloride in the aqueous solutions of cocamidopropyl betaine (CAPB) on the properties is the opposite to the one described in the literature for cationic and anionic surfactants, i.e., CMC increases with increasing ionic strength, foam height decreases with increasing salt concentration. Their investigation showed that sodium chloride makes worse the properties of the CAPB solutions [18].
3. Results
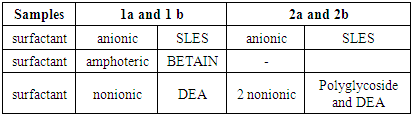
According to the type of selected surfactants for the preparation of liquid soap samples, all samples can be divided into two groups. The first group samples were labeled as 1a and 1b, while the second group as 2a and 2b. (Table 1).
3.1. The Surfactant Constitution of Liquid Soap
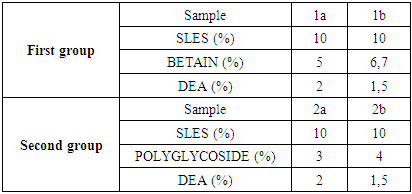
The table 1 shows the different ingredients of surfactants in two groups of experiments.The first group of samples contain: anionic surfactant (SLES), amphoteric surfactant (BETAIN) and nonionic surfactant (DEA). The mass fraction of anionic surfactant was kept constant, while the mass ratios of amphoteric and nonionic surfactants were changed (Table 1 and Table 2).Table 1. The surfactant constitution of samples
 |
| |
|
Table 2. The constitution of samples regarding type and mass percent of surfactants
 |
| |
|
The second set of samples was made of: anionic surfactant (SLES), and two nonionic surfactants, polyglycoside and DEA. The mass fraction of anionic surfactant was kept constant, while the mass ratios of nonionic surfactants were changed. The other ingredients we keep constant for all experiment (Table 1 and Table 2).
3.2. Physicochemical Properties of Liquid Soap
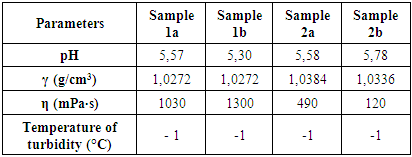
In order to investigate the influence of surfactants on the physicochemical properties, (viscosity, surface tension, pH, electrical conductivity, and density) for each surfactant in different concentration range were determined. It is also examined what is the optimal surfactant ratio (mass %) as well as the combination of surfactants in terms of their charge. The influence of tensile charge on the medium in which it is present and the hydrophobic/hydrophilic interaction of surfactant/medium were investigated. The stability of the resulting liquid soap in the dark and under the influence of UV/Vis light, followed by thermal stability at three examined temperatures, followed by color, fragrance and appearance were monitored during three months.The parameters such as pH value, specific weight (γ), viscosity (η), temperature of turbidity of each prepared liquid soap were determined. The results are shown in table 3.Table 3. pH value, specific weight (γ), viscosity (η), temperature of turbidity for all prepared samples
 |
| |
|
3.2.1. Effect of Surfactant Mixture on pH Value of Liquid Soap
In plot 1 is shown the pH values of four different prepared samples of liquid soaps.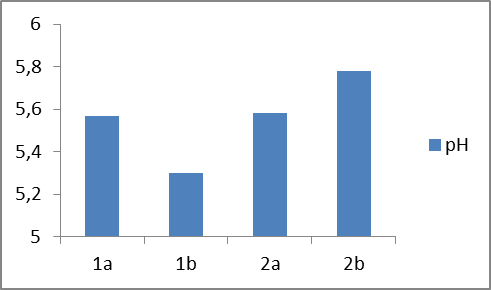 | Plot 1. Show the pH of four different types of liquid soaps |
3.2.2. Effect of Surfactant Mixture on Specific Weight
In plot 2 are shown the specific weight of four different prepared samples of liquid soaps.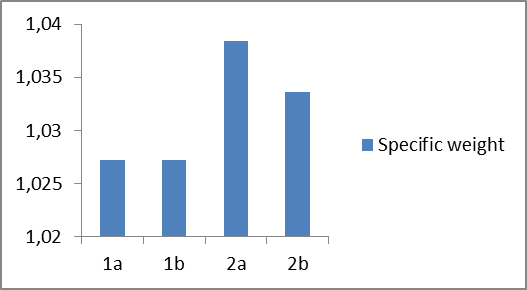 | Plot 2. Show the specific weight of four different types of liquid soaps |
3.2.3. Effect of Surfactant Mixture on Viscosity
In plot 3 are shown the viscosity, η (mPa·s) of four different types of liquid soaps-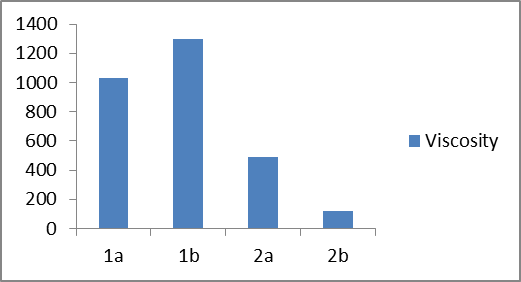 | Plot 3. Show the viscosity, η (mPa·s) of four different types of liquid soaps |
3.2.4. Study of Surface Tension
The surface tension of water at the air–water interface decreases to a minimum value and becomes almost constant or it may show a slight increase in this value with the increase in concentration of the surfactant solution, causing improved adsorption ability at the interface with the increase in concentration [19].The sharp decrease in the value of surface tension indicates that the surfactant molecules have greater affinity towards the air–water interface. The specific concentration value at which the surfactant molecules are completely adsorbed at the air–water interface, thus favoring the formation of micelles, is called the critical micelle concentration (CMC).Critical micelle concentration (CMC) values were obtained by surface tensiometry measurements. The surface tension at the air–water interface of the surfactant solutions with varying concentrations were determined using a digital tensiometer Digital - Tensiometer K10T Krüss. Surface tension values for each individual sample were measured three times to maintain the consistency of the results, and the final recorded values were taken as the average of the measured values. After measuring each set of different concentrations, the platinum ring used in the experiment was cleaned using acetone and was dried in air for the next set of experiments.In table 4 are shown the values of surface tension (mN/m) for different concentrations (mol/L) of certain surfactants in order to determine CMC.Table 4. Surface tension vs surfactant concentration at 23°C
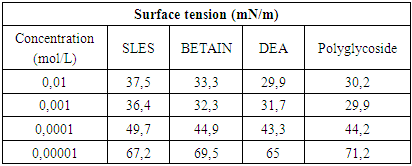 |
| |
|
In the plot 4 is presented the surface tension isotherms for aqueous surfactant solutions.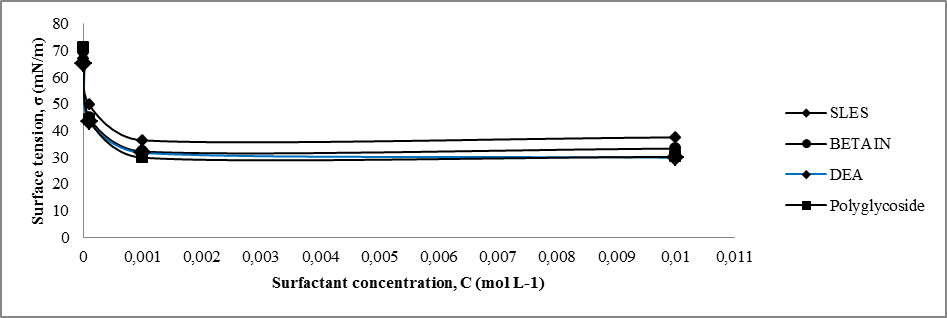 | Plot 4. Surface tension isotherms for different types of aqueous surfactant solutions |
3.2.5. Study of Electrical Conductivity
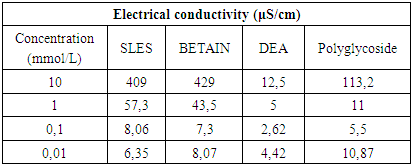
Electrical conductivity studies were conducted to determine the CMC of the surfactant system. The electrical conductivities of the surfactant solutions with varying concentrations were determined using a pH/Conductometer Mettler Toledo MPC 227 equipped with Conductometric Electrode Mettler Toledo InLab 730 NTC, 0 ... 1000 mS, 0 ... 100°C.The effects of concentration of surfactants on the electrical conductivity are shown in Table 5 and Plot 5.Table 5. Electrical conductivity vs surfactant concentration at 23°C
 |
| |
|
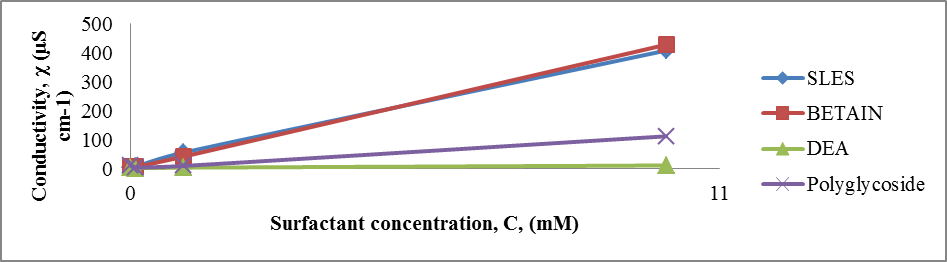 | Plot 5. Electrical conductivity versus surfactant concentration |
The electrical conductivity of a solution depends on the mobility and number of ions or charged particles present in the solution. In the case of ionic surfactants, the electrical conductivity increases upon increasing the surfactant concentration. The formation of micelle is effected on conductivity of ionic solution.
3.2.6. Study of Density
The density of the prepared surfactants solutions were determined simply by using a pycnometer.
3.2.7. Determination of Stability by Monitoring of Appearance, Color and Fragrance
Parameters such as appearance, color and fragrance were monitored during three months at 3 different temperatures at +4°C, room temperature, +40°C, but also in the dark and under UV light for 3 months. Every week these parameters were determined. After 45 days we have notice some mild changes in color and fragrance at 40°C and under UV light.
4. Discussion
Surfactants hold certain beneficial properties, their use in everyday life becomes nearly indispensable. They have applications not only in skin cleansers, but also in the cosmetic, paint, pesticide, textile industries, and even food; however, the irritation potential of surfactants may relatively limit their employment. Therefore, development of less irritant, consumer-friendly surfactants or mixed surfactant detergents systems are of general interest.
4.1. Effect of Surfactant Mixture on pH, Specific Weight and Viscosity of Liquid Soap
Based on the experimental results of the first group of samples (1a and 1b), it can be seen that in sample 1b with an increase in concentration of amphoteric surfactant (BETAIN) by 1.7% and by decreasing concentration of nonionic surfactant (DEA) by 0.5%, leading to change of pH values from 5.57 to 5.30 (Plot 1), while the specific weight remains constant (Plot 2), but viscosity increases from 1030 to 1300 mPas (Plot 3).When comparing the experimental results of the second group of samples (2a and 2b) it can be observed that in the sample 2b with increasing of the concentration of the non-ionic surfactant polyglycoside by 1% and reducing of the concentration of nonionic surfactant (DEA) by 0.5%, a mild increase of the pH value from 5, 58 to 5.78 (Plot 1), while the specific weight remains constant (Plot 2), but there is a decrease in viscosity from 490 to 120 mPas (Plot 3). On the other side, we can notice significant changes in specific weight and viscosity comparing first and second group of experiments that is with changing the surfactants type and mass ratio. We can notice an increase in specific weight from first to second group of samples and decrease in viscosity. When comparing the influence of the surfactant on the physicochemical parameters of the obtained liquid soaps, it can be concluded that the greatest influence is reflected on the change of viscosity and it ranges from the first to the second group of samples from (1030-1300) to (120-490), while the pH changes and the specific weight is negligible.
4.2. Determination of Critical Micelle Concentration by Studying of Surface Tension, Electrical Conductivity and Density
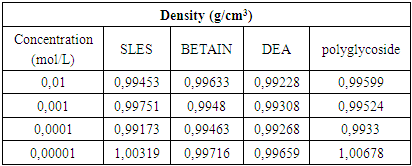
From the experimental data obtained for surface tension (Table 4 and Plot 4), electrical conductivity (Table 5 and Plot 5) and density (Table 6 and Plot 6) it can be seen that the critical micelle concentration was in the range of 1x10-4 – 1x10-3 mol/L of all surfactant types. All these measurements were done at 23°C (room temperature).Table 6. Density vs surfactant concentration at 23°C
 |
| |
|
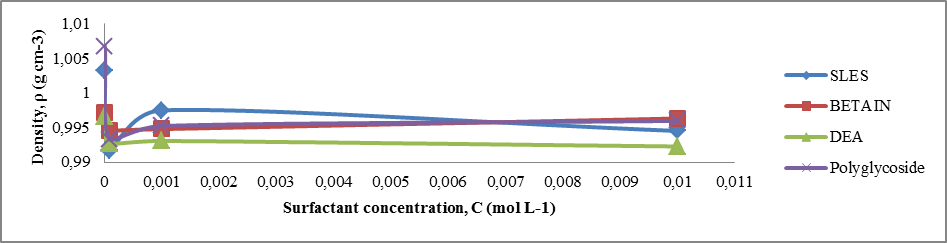 | Plot 6. Density of surfactants for different concentrations |
From experimental data in Table 4 and Plot 4 it can be seen that surface tension is lower for both nonionic surfactants comparing to zwitterionic (amphoteric) and anionic surfactants.From experimental data in Table 5 and Plot 5 it can be seen that nonionic surfactants has the lowest electrical conductivity comparing to zwitterionic and anionic surfactant. This is in accordance with theoretical knowledge since that nonionic surfactants do not have any charge and that they do not ionize. In the case of ionic surfactants, in this case anionic and amphoteric, the electrical conductivity increases upon increasing the surfactant concentration. It is important that ionization state of the zwitterionic surfactant is depended on pH solution.Even by determination of density for different surfactant solution can be seen that significant change is in the range of critical micelle concentration, in the concentration range of 1x10-4 – 1x10-3 M.The influence of type of surfactant and its mass concentration was evaluated with an objective to understand physiochemical changes firstly viscosity of prepared liquid soaps and then to study each single surfactant and to determine the critical micelle concentration. All physicochemical parameters change in this concentration range. It can be concluded that the first group of samples is much closed to the qualities of a commercial liquid soap. The additional value of this process of synthesis is that this liquid soap containing a glycerin which is well known as a nus product in the production of biodiesel. So in this context, we can also speak about sustainability of this procedure for preparation of liquid soap. The present investigation highlights the importance of chosen surfactant compounds and other constituents on physicochemical properties. This procedure surely can be a good starting point for a new investigation of new ways in production of liquid soaps.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the DITA 1977 detergent industry in Tuzla, Bosnia and Herzegovina which has given us the support in using their equipment and chemicals for research.
References
| [1] | Reyhaneh Azarmi and Ali Ashjaran, Type and application of some common surfactants, Journal of Chemical and Pharmaceutical Research, 2015, 7(2): 632-640. |
| [2] | L. L. Schramm, Surfactants: Fundamentals and Applications in Petroleum Industry, Cambridge University Press, Cambridge, 2000. |
| [3] | Anita Bocho-Janiszewska, Tomasz Wasilewski, Application of Glycerin in Liquid Laundry Detergents as an Example of Innovation in the Household Chemicals Industry, Tenside Surf. Det. 54 (2017) 5, DOI: 10.3139/113.110517. |
| [4] | Attwood D, Florence AT. Surfactant systems; Their Chemistry, Pharmacy and Biology. London: Chapman & Hall, 1983. |
| [5] | Reynolds JEF. Martindale: the extra pharmacopoeia, 32nd edn. Royal Pharmaceutical Society, London, 1999. |
| [6] | M. J. Rosen, Surfactants and Interfacial Phenomena, John Wiley and Sons, Inc. 3rd Ed, New Jersey, 2004. |
| [7] | Louis HO TAN TAI, Véronique NARDELLO-RATAJ, The main surfactants used in detergents and personal care products, Detergents, Oléagineux, Corps Gras, Lipides. Volume 8, Numéro 2, 141-4, Mars - Avril 2001, Dossier: Tensioactifs: savons et detergents. |
| [8] | Effendy I, Maibach HI., Surfactants and experimental irritant contact dermatitis. Contact Dermatitis, 1995; 33: 217 ± 225. |
| [9] | Reyhaneh Azarmi and Ali Ashjaran, Type and application of some common surfactants, Journal of Chemical and Pharmaceutical Research, 2015, 7(2): 632-640. |
| [10] | Zografi, G, Schott H, Swarbrick J. Interfacial phenomena In: Gennaro AR (ed) Remington’s Pharmaceutical Sciences. Mack, Easton, PA, 1990; pp 257–272. |
| [11] | McBain JW, Cornish ECV, Bowden RC (1912) Studies of the constitution of soap in solution: sodium myristate and sodium laurate. J. Chem. Soc., Trans., 101: 2042–2056. |
| [12] | Cudina O, Karljikovic-Rajic K, Ruvarac-Bugarcic I, Jankovic I (2005) Interaction of hydrochlorothiazide with cationic surfactant micelles of cetyltrimethylammonium bromide Colloids Surf., A, 256: 225–232. |
| [13] | Hosseinzadeh R, Gheshlagi M (2009) Interaction and micellar solubilization of diclofenac with cetyltrimethylammonium bromide: a spectrophotometric study., Collect. Czech. Chem. Commun., 74 (3): 503–513. |
| [14] | L. L. Schramm, E. N. Stasiuk, D. Gerrard Marangoni, Annu. Rep. Prog. Chem., Sect. C, 99, 3, 2003. |
| [15] | A. W. Adamson, A. P Gast, Physical Chemistry of Surfaces, 6th Ed., John Wiley & Sons, New York, 1997. |
| [16] | E. Pramauro, E. Pelizzetti, Surfactants in Analytical Chemistry: Applications of Organized Amphiphilic Media, Elsevier, Amsterdam, 1996. |
| [17] | Sharon E. Jacob and Sadegh Amini, Cocoamidopropyl Betaine, Dermatitis 19(3):157-160, 2008, DOI: 10.2310/6620.2008.06043. |
| [18] | Katarzyna Staszak, Daria Wieczorek, Katarzyna Michocka, Effect of Sodium Chloride on the Surface and Wetting Properties of Aqueous Solutions of Cocamidopropyl Betaine, J Surfact Deterg (2015) 18:321–328, DOI 10.1007/s11743-014-1644-8. |
| [19] | Neha Saxena, Nilanjan Pal, Keka Ojha, Swapan Dey, and Ajay Mandal, Synthesis, characterization, physical and thermodynamic properties of a novel anionic surfactant derived from Sapindus laurifolius, RSC Adv., (2018), 8, 24485, DOI: 10.1039/c8ra03888k. |








 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




