-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2018; 8(2): 23-30
doi:10.5923/j.ijmc.20180802.01

Removal of Dyes from Waste Waters through Adsorption Using HCl Activated Coal
Misael Silas Nadiye-Tabbiruka, Lebogang Lungani
Chemistry Department, University of Botswana, Gaborone, Botswana
Correspondence to: Misael Silas Nadiye-Tabbiruka, Chemistry Department, University of Botswana, Gaborone, Botswana.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Purification of waste water by removal of methylene blue using acid activated Morupule coal was investigated through a study of thermodynamics and kinetics of the dye adsorption from aqueous solution at various concentrations, pH and temperature. The rate of adsorption and quantity adsorbed were found to increase with increasing initial methylene blue concentration, decreasing pH and increasing temperature. Adsorption followed a two stage profile indicating an increase in available surface for the process probably initiated by initial adsorption causing swelling of the coal. Kinetics data follows the Legergren pseudo second order model best indicating the existence of two simultaneous adsorption processes thought to be adsorption on the outer and inner surfaces as well as on the surface freshly exposed by swelling. The thermodynamics data fitted the Freundlich model best indicating heterogeneity of the coal surface.
Keywords: Dyes removal, Waste water purification, Dyes’ adsorption, Kinetics and thermodynamics
Cite this paper: Misael Silas Nadiye-Tabbiruka, Lebogang Lungani, Removal of Dyes from Waste Waters through Adsorption Using HCl Activated Coal, International Journal of Materials and Chemistry, Vol. 8 No. 2, 2018, pp. 23-30. doi: 10.5923/j.ijmc.20180802.01.
Article Outline
1. Introduction
- Industries such as textile, paper, leather plastic to mention a few, play an important role in economic development. However, the disposal of waste waters from these industries into the environment is a cause for great concern [1]. Textile waste waters are highly coloured by many types of toxic organic dyes which are of great threat to human’s health and to aquatic organisms. Most of these highly coloured industrial waste water dyes are stable to light and are not biodegradable either. Their presence in water decreases light permeability to aquatic plants and organisms affecting photosynthesis [2]. Therefore removal of such dyes from industrial effluent waters is of interest [1].Various methods of removing dyes from textile waste waters have been studied. They include electro chemical, coagulation, oxidising agents, precipitation, photo catalysis, flocculation and adsorption [2, 3, 4, 5] to mention but a few. Adsorption is considered as the preferred method because it is cheap, easy to carry out and adsorbent materials are abundant [2]. This technique depends on high specific surface area, porosity and narrow particle size distribution of the adsorbent [6]. A variety of activated carbons have been used as adsorbents in many studies because of their large adsorption capacity. Activated carbons can be highly porous and can have high surface area to volume ratio. Example of these carbons include coir pith carbon [7], Periwinkle shells [8], Bamboo dust, coconut, ground nut shell, rice husk and straw [9], scrap tyres, bituminous wastes and coal [10].Various water purification technics and processes have recently received attention. In some cases single technics while in others combinations of technics have been applied in multivariate approaches to remove and in some cases to recover useful impurities. A coagulation and flocculation process has been used to remove and recover silver from industrial waste water in the mirror factories [11] An initial attempt to explore the possibility and viability of applying microbial fuel cells (MFC) as a sustainable technology for a large scale waste water treatment was critically examined by Wen-Wei Li et al [12]. They conclude that the way forward for MFC, its hybrids and its combinations with other technics requires further investigation producing statistical validation data. In order to enable in situ solvent and reagent recycling, Tamas Fodi et al [13] coupled a nano filtration unit to a continuous flow reactor. This resulted in a solution of the product 11 times more concentrated than the reaction mixture and massively increased purity. Work on separation and purification using membranes has been investigated ranging from triple layer hollow fibre catalytic membrane reactor for the production of hydrogen [14] which was found to work better than the usual fixed bed reactor, to reclamation of sodium sulphate from industrial waste water by using membrane distillation and membrane crystallisation technics thus generating fresh water and avoiding industrial waste disposal [15].In this study coal obtained from Morupule colliery was activated using hydrochloric acid (HCl) and was used as an adsorbent for removing methylene blue from its aqueous solution. This coal is currently only used in thermal power stations for generating electricity. Therefore using the coal for waste water treatment will be an added advantage.Methylene blue (fig.1), one of the dyes used in textile industries for dyeing cloth materials, causes eye burns which may lead to permanent injury whilst inhalation can result in difficulty in breathing [1]. Furthermore, Ingestion of the dye leads to symptoms such as burning sensation, vomiting, nausea and diarrhoea [16].
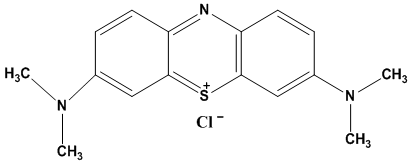 | Figure 1. Structure of methylene blue |
2. Experimental
2.1. Materials and Methods
2.1.1. Morupule Coal
- Coal sample were collected from different parts of Morupule mine and mixed to give a representative sample. Samples from this mixture were ground into small particles using a motor and a pestle and then heated in an oven at 423 K for an hour to facilitate thermal decomposition and to remove volatile compounds. They were cooled and stored in a desiccator for further use.Activation The coal was activated using an edited method adapted from that of Zhang et. al. [17]; 50 g ground coal was mixed with 200 ml 2 M hydrochloric acid (HCl) and the mixture was placed in a shaker water bath at 373 K for two hours. The mixture was filtered using whatmann filter paper. The residue particles were washed with several portions of distilled water to remove the acid, dried in an oven at 323 K for two hours, cooled and then stored in an air tight bottle for further use.
2.1.2. Methylene Blue
- Methylene blue supplied by Thembane chemicals South Africa was used as the adsorbate. A stock solution of 0.001M was prepared using distilled water. Five standards of concentrations 2 x 10-5 M, 4 x 10-5 M, 6 x 10-5 M, 8 X 10-5 M and 1 x 10-4 M were prepared from the stock solution. The standards were used to study the kinetics and thermodynamic of adsorption of the methylene blue onto hydrochloric acid activated Morupule coal.
2.2. Equipments
2.2.1. Ultra Violet-Visible (UV-Vis) Spectrophotometer
- A double beam recording UV-Vis spectrophotometer was used to determine the exact concentration of methylene blue, from the measured absorbance of the filtrate from the reaction mixture.
2.2.2. Calibration of the Spectrophotometer
- Absorbances of six standard solutions of methylene blue were measured at wavelength of 664 nm. A calibration curve of absorbance against concentration was made and was used to obtain the exact concentration values of methylene blue solutions during or after adsorption.
2.3. Procedures
2.3.1. Adsorption Kinetics
- In each experiment, an accurately weighed amount of activated coal (1g) was added to a 100 ml reaction bottle containing 50 ml of methylene blue. The mixture was agitated in a labcon shaking water-bath at a speed of 100 rpm at constant temperature (298 K). Samples of 5 ml of the mixture were collected after various time intervals for a period of 2 hours, centrifuged, filtered using Whatmann filter papers and their absorbance measured using the calibrated UV-VIS spectrophotometer at a predetermined wavelength of 664 nm. This procedure was repeated several times for all concentrations in the kinetics study. The values used in analysis were obtained from a smooth curve fitted in the data. The concentrations of methylene blue were obtained from the measured absorbance from a calibration curve and used to calculate the amount of methylene blue adsorbed onto the activated coal using the following equation;
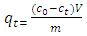 | (1) |
2.3.2. Adsorption Thermodynamics
- A batch method was used to obtaining adsorption isotherms, 1 g of activated Morupule coal was added to each of the five 100 ml reaction bottles containing 50 ml of different initial methylene blue concentrations (2 x 10-5 M, 4 x 10-5 M, 6 x 10-5 M, 8 x 10-5 M and 1 x 10-4). The bottles were kept in a shaking water bath at a speed of 100 rpm for a predetermined period (2 hrs) at 298K. The experiments were repeated several times to ensure reproducibility. The equilibrium concentrations of the dye were analyzed using a uv/vis spectrophotometer as described in kinetics analysis. The amount of dye adsorbed at equilibrium was calculated using the equation below;
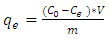 | (2) |
3. Results and Discussion
3.1. Effects of Contact Time
- Fig 2 shows that two equilibria observed for all concentrations except for the lowest (2 x 10-5 M). The initial adsorption was rapid reaching the first equilibrium within the first 5 minutes of agitation. The first equilibrium was maintained for about thirty minutes and then a second sudden rapid increase was observed. After about 70 minutes depending on the initial concentration the adsorption process slowed down reaching a second equilibrium thereafter. The first rapid uptake of the dye is thought to be due to the availability of easily accessible active sites on the external surface of the adsorbent. The second uptake is thought to be due to adsorption of methylene blue in the pores constituting the internal surface area of the coal [9] and the additional area exposed by swelling caused by the initial methylene adsorption.
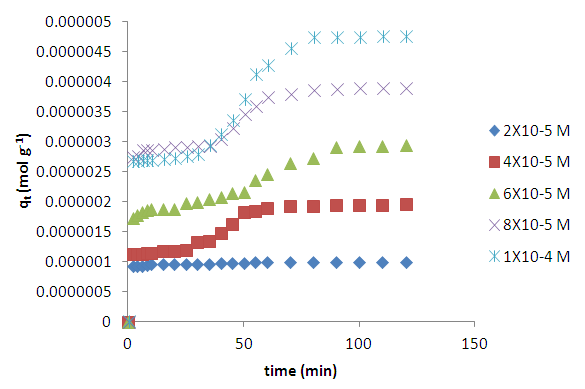 | Figure 2. Effects of contact time and varying initial concentration on the adsorption of methylene blue onto HCl activated coal at 298 K |
3.2. Effects of Initial Dye Concentration on Adsorption Kinetics
- Fig. 2 shows the effect of initial dye concentration on the adsorption of the dye onto acid activated coal. The amount of methylene blue dye adsorbed increases with increase in initial dye concentration with the profile for concentrations higher than 2x10-5M showing a two stage process. It is thought that the first pseudo equilibrium is due to adsorption on the external surface of the coal originating from the easy availability of active sites for adsorption on the adsorbent [18]. This part of the process is instantaneous on the very dilute solution. More concentrated dye solutions take longer time to reach final equilibrium as compared to lower concentrated solutions as a second step took over from the first pseudo equilibrium. It is thought that at higher concentrations the initial adsorption leads to swelling of the adsorbent thus exposing the inner surface and pores for more adsorption. This part of the process will probably be slower due to diffusion of this large molecule into this hidden surface in the pores of the adsorbent [1].
3.3. Effects of Temperature on Adsorption Kinetics
- Fig 3 shows results from investigations of effects of temperature on the adsorption of methylene blue at temperatures of (298, 308, 318 and 328 K). It is clear that the amount of dye intake increased with increase in temperature. This would point to activated adsorption and hence the possibility of chemisorption. However, coal is known to swell on heating, thus exposing hidden surface area for further adsorption. Studies on desorption [26] have shown a decrease in amount adsorbed with decreasing concentration, thus pointing to physisorption.
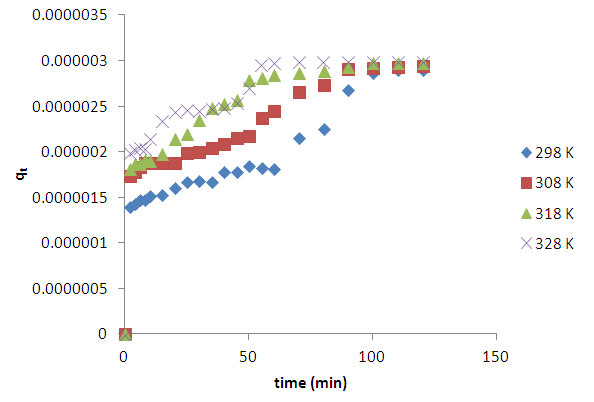 | Figure 3. Effect of variation of temperature on the adsorption of methylene blue (6 x 10-5 M) onto HCl activated coal |
3.4. Effects of pH
- The effects of pH on the adsorption of methylene blue from solution onto acid activated coal are shown in figure 4. Once again the two equilibrium stages are clear and the tendency to merge to give one adsorption capacity is visible. It clearly shown that methylene blue adsorption is favoured mostly by acidic conditions as compared to neutral and basic medium giving an adsorption velocity profile trend pH2>pH3>pH7, pH8 and pH9. Clearly, the pH of a dye solution has a significant effect on the adsorption process probably due to the changes on the surface charges and changes on the adsorbate molecule [18].
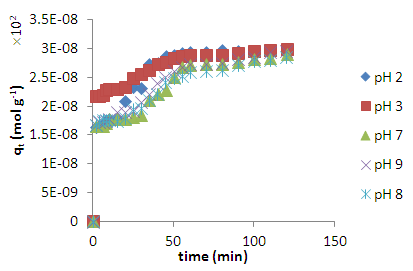 | Figure 4. Effect of pH on the adsorption kinetics of methylene blue (6 x 10-5 M) at 298 K on HCl activated coal |
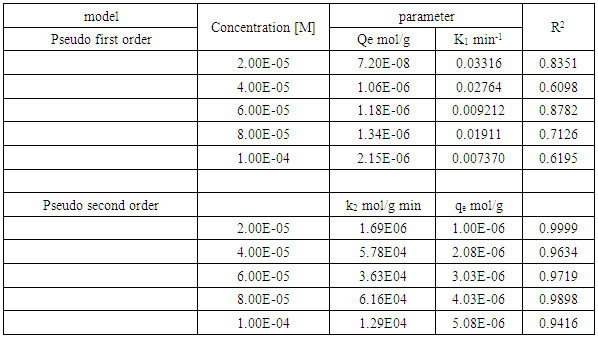
3.5. Analysis of Adsorption Kinetics Results
- The data obtained from kinetic studies were fitted to the Lagergren pseudo first and second order models to obtain a clear understanding of the interaction between the acid activated coal and methylene blue [19] using the linear equations shown below.
3.5.1. Pseudo First Order Model
- Lagergren pseudo first order is given by:
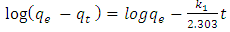 | (3) |
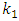 is the pseudo first order rate constant in min-1,
is the pseudo first order rate constant in min-1, 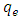 is the amount adsorbed at equilibrium,
is the amount adsorbed at equilibrium, 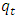 is the amount adsorbed at time t and t is the time. The validity of Pseudo first order model was checked by obtaining linear plots of log (qe-qt) against t.The linear plot of pseudo first order (fig 5) was used to obtain the rate constants of pseudo first order (k) and amount adsorbed at equilibrium (qe). The obtained values including the correlation coefficients (R2) are shown in table 1. The fitting is poor, the correlation coefficients obtained from all the linear plots are way too far from unity implying that the adsorption process does not follow the model.
is the amount adsorbed at time t and t is the time. The validity of Pseudo first order model was checked by obtaining linear plots of log (qe-qt) against t.The linear plot of pseudo first order (fig 5) was used to obtain the rate constants of pseudo first order (k) and amount adsorbed at equilibrium (qe). The obtained values including the correlation coefficients (R2) are shown in table 1. The fitting is poor, the correlation coefficients obtained from all the linear plots are way too far from unity implying that the adsorption process does not follow the model.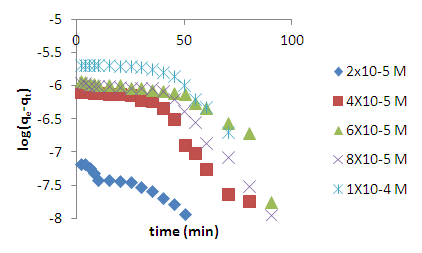 | Figure 5. Lagergren pseudo first order model plot for methylene blue adsorption onto HCl activated coal at 298 K |
3.5.2. Pseudo Second Order Model
- Lagergren pseudo second order equation is given by:
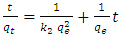 | (4) |
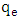 is the amount adsorbed at equilibrium,
is the amount adsorbed at equilibrium, 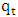 is the amount adsorbed at time t and t is the adsorption time.Linear plot were obtained for the pseudo second order model (fig 6). The pseudo second order rate constants (k) and amount adsorbed at equilibrium (qe) were determined using the linear plots and are given in table 1. The correlation coefficients obtained from the plot are closer to unity implying that the adsorption of methylene blue onto acid activated Morupule coal follows pseudo second order kinetics model [20]. However, this implies that the process is actually third order with one of the reactant in excess. Once again this means that at the rate determining step, a mechanism involving collision of three bodies occurs. The probability of such a collision is very small and hence it is an unlikely mechanism. However two simultaneous two body collisions are also known to fit the equation. In this current investigation these would probably be a simultaneous adsorption on the external surface and on the internal surface in the pores particularly those exposed by gradual swelling. Earlier work on nano coal clay composites [21] has shown that indeed the process reverts to pseudo first order kinetics on coal miniaturisation.
is the amount adsorbed at time t and t is the adsorption time.Linear plot were obtained for the pseudo second order model (fig 6). The pseudo second order rate constants (k) and amount adsorbed at equilibrium (qe) were determined using the linear plots and are given in table 1. The correlation coefficients obtained from the plot are closer to unity implying that the adsorption of methylene blue onto acid activated Morupule coal follows pseudo second order kinetics model [20]. However, this implies that the process is actually third order with one of the reactant in excess. Once again this means that at the rate determining step, a mechanism involving collision of three bodies occurs. The probability of such a collision is very small and hence it is an unlikely mechanism. However two simultaneous two body collisions are also known to fit the equation. In this current investigation these would probably be a simultaneous adsorption on the external surface and on the internal surface in the pores particularly those exposed by gradual swelling. Earlier work on nano coal clay composites [21] has shown that indeed the process reverts to pseudo first order kinetics on coal miniaturisation.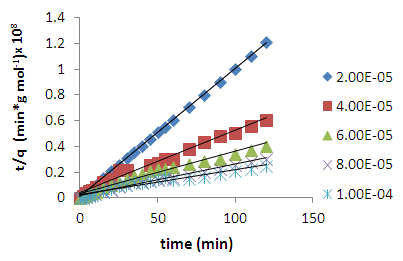 | Figure 6. Lagergren pseudo second order model plot for methylene blue adsorption onto HCl activated coal at 298 K |
|
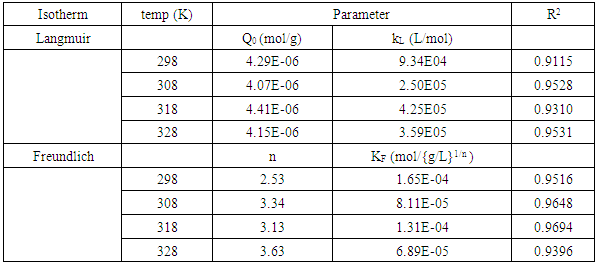
3.6. Analysis of Results from Thermodynamics Study
- Adsorption isothermsAdsorption isotherms describe how the adsorbate molecules interact with adsorbent molecules, and describe the distribution of the adsorbate species among liquid and adsorbent at equilibrium [22]. These isotherms include the Langmuir and Freundlich linear isotherms and were used to describe the equilibrium nature of adsorption of methylene blue onto HCl activated Morupule coal.
3.6.1. Langmuir Isotherm
- The Langmuir isotherm model assumes that adsorption is limited to monolayer and also that adsorption occurs on homogeneous sites within the adsorbent [23]. The Langmuir isotherm [15] is represented by the following linear equation;
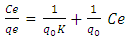 | (5) |
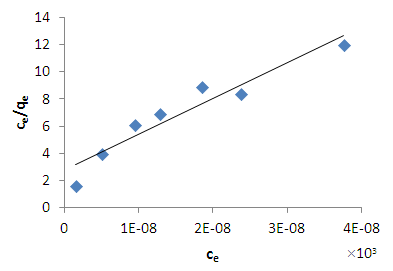 | Figure 7. Langmuir isotherm for methylene blue adsorption onto HCl activated coal at 298 K |
3.6.2. Freundlich Isotherm
- The data from the thermodynamic study was also fitted onto the Freundlich isotherm. This is an empirical relation between the concentrations of a solute on the surface of an adsorbent to the equilibrium solute concentration and assumes a varying surface distribution of adsorption sites [20]. The linearized form of Freundlich model is given below;
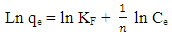 | (6) |
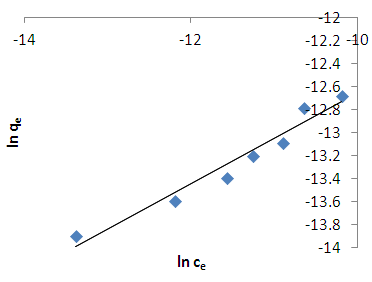 | Figure 8. Freundlich isotherm for methylene blue adsorption on HCl activated coal at 298.15 K |
|
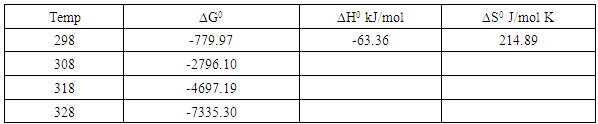
3.6.3. Thermodynamic Parameters
- The Gibbs free energy change (ΔG0), entropy change (ΔS0) and enthalpy change (ΔH0) for adsorption of methylene blue onto HCl activated Morupule coal were obtained using the equations below and are shown in table 3.
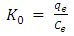 | (7) |
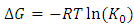 | (8) |
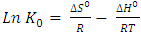 | (9) |
|
3.7. Comparison of Acid Activated Coal with Commercial Activated Carbon
- The kinetics of adsorption of methylene blue by our HCl activated coal is compared with the kinetics of adsorption by commercial activated carbon in figure 8. It is clear that the HCl activated coal has a capacity for methylene blue similar to that of the commercial activated carbon. However, the HCl activated coal is slightly slower in getting to its maximum capacity. It is believed that this is due to the fact that the acid activated coal has to swell in order to avail the hidden area to the adsorbate. It has been shown by Sejie [21] that miniaturisation to nano particles eliminates swelling but only improves adsorption capacity by a small amount. It was shown by Unaye [27] in earlier work that chemical activation of coal using zinc chloride increased the specific surface area from 10 m2/g to 280 m2/g. This method however leads to pollution by zinc chloride waste and requires heating to high temperatures hence wastes energy. Acid activation is cheap and simple, requires minimal heating and does not pollute, as HCl, citric or acetic acid can be used. This method is therefore suitable for small scale activation industry, domestic water purification for domestic consumption as well as to decolourise industrial effluent water.
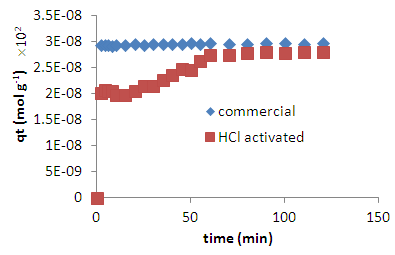 | Figure 9. Comparison of adsorption kinetics and capacity of the HCl activated Morupule coal with commercial activated charcoal |
4. Conclusions
- In this study the thermodynamics and kinetics of methylene blue removal from its aqueous solution through adsorption onto hydrochloric acid activated Morupule coal was successfully conducted. The dye uptake increased with increase in dye concentration and temperature. The equilibrium data fitted the Freundlich model better than the Langmuir isotherm implying that the HCl activated Morupule coal has an energetically heterogeneous surface. The data obtained from the kinetics study fitted the pseudo second order model better than the pseudo first order indicating that two processes were occurring simultaneously. These were identified as adsorption on the external surface and adsorption on the inner surface in the pores together with onto the surface exposed by the gradual swelling caused by the initial adsorption. A small negative value of ∆H0 (-63.4 kJmol-1) was obtained confirming an exothermic physical adsorption process while a positive ∆S0 (0.215kJmol-1) confirmed the affinity of the activated Morupule coal for methylene blue.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge contributions from the Department of Chemistry for the materials, and space to perform the various experiments, Morupule Colliery for the coal samples used in this research and the University of Botswana for the funding of the project.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML