-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2018; 8(1): 10-14
doi:10.5923/j.ijmc.20180801.02

A Study of the Absorption Edge of CdS:In Thin Films Prepared by the Spray Pyrolysis Technique
Shadia J. Ikhmayies
Al Isra University, Faculty of Information Technology, Department of Basic Sciences-Physics, Amman, Jordan
Correspondence to: Shadia J. Ikhmayies, Al Isra University, Faculty of Information Technology, Department of Basic Sciences-Physics, Amman, Jordan.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cadmium sulphide (CdS) is a II-VI compound with a wide band gap energy that enables it to be used as a window material in the CdTe based solar cells. In this work the absorption edge of indium doped cadmium sulphide (CdS:In) thin films was investigated. The films were prepared on glass substrates using the spray pyrolysis technique at a substrate temperature Ts = 490°C. X-ray diffraction (XRD) and scanning electron microscopy (SEM) revealed that the films are polycrystalline. The films displayed a wurtzite hexagonal structure with some indications of the presence of the cubic zinc blind phase. The transmittance of the films was measured at room temperature in the wavelength range 400–1100 nm and used to deduce the absorbance. The second derivative of the absorbance revealed the existence of the free exciton peaks of the wurtzite phase. The A and B free exciton peaks showed a merged peak and the C free exciton peak was apparent. These results were compared with those obtained by different authors. The separations of the aforementioned peaks were deduced and found to be in good agreement with the values found in the literature.
Keywords: Cadmium sulphide, Thin films, X-ray diffraction, Absorption edge, Exciton peaks, Heterojunction solar cells
Cite this paper: Shadia J. Ikhmayies, A Study of the Absorption Edge of CdS:In Thin Films Prepared by the Spray Pyrolysis Technique, International Journal of Materials and Chemistry, Vol. 8 No. 1, 2018, pp. 10-14. doi: 10.5923/j.ijmc.20180801.02.
1. Introduction
- Cadmium sulphide CdS is a wide band gap II-VI compound semiconductor. It has direct band gaps (Eg) of 2.57 and 2.40 eV, at room temperature for hexagonal and cubic structures respectively [1]. CdS films have a great application potential such as the use in optoelectronic devices and solar cells [2, 3]. CdS is used as heterojunction partner for polycrystalline CdTe to fabricate CdTe/CdS solar cells, where a CdS layer is used as the n-type window layer [3]. To take advantage of the optoelectronic properties of CdS by producing n-type films, extrinsic doping is required. Several methods have been developed to grow n-type CdS films: for example; the variation of the reagent precursors concentration in order to vary the Cd:S ratio, or the doping with silver [4], chlorine [5] or indium atoms [6-9]. In this work indium was used as a dopant to produce n-type CdS thin films.There are different techniques that can be used to obtain CdS thin films, such as rf sputtering [10], close spaced vapor transport (CSVT) [11], chemical bath deposition (CBD) [3, 12], close-spaced sublimation (CSS) [12], pulsed laser deposition (PLD) [13, 14], and spray pyrolysis (SP) [15-33]. However, the SP technique is a very low cost and simple technique that enables intentional doping and getting large area and uniform thin films.In this work, the valence-band splitting of the wurtzite CdS:In thin films arising from the crystal field could be observed in the second derivative of the absorbance in the region of the absorption edge. The maxima related to the free exciton peaks of the wurtzite structure of CdS:In thin films were apparent and their positions and separations were deduced and compared with those obtained by other authors.
2. Experimental Procedure
- The films were prepared by the spray pyrolysis (SP) technique on glass substrates as described elsewhere [15-19]. The dimensions of the slides are (6cm × 2.6 cm × 0.1 cm) and the substrate temperature is 490°C. Approximately a stoichiometric solution of thiourea ((NH2)2CS) and cadmium chloride (CdCl2.H2O) in distilled water was used. Indium chloride (InCl3) was used as the source of indium, where the ratio of the concentration of indium ions to that of cadmium ions ([In]/[Cd]) in the sprayed solution was 10-2. X-ray measurements were recorded by a Philips PW1840 Compact X-ray diffractometer system with Cu Kα (λ = 1.5405 Å). Both of the SEM micrographs and compositional analysis were taken by a FEI scanning electron microscope (Inspect F 50), which is supplied by X-ray energy dispersive spectroscopy (EDS) apparatus.Transmittance of the films was measured by using a double beam Shimadzu UV 1601 (PC) spectrophotometer with respect to a piece of glass similar to the substrates in the wavelength range 400-1100 nm. The thickness of the films was estimated by using the transmission spectra of the films and Lambert law of absorption in a semiconductor.
3. Results and Discussion
- Fig.1 shows the X-ray diffractogram (XRD) of one of CdS:In thin films prepared in this work. As seen in the figure the film is polycrystalline and it displays a predominantly wurtzite hexagonal structure. Weak lines of the zinc-blind phase can be observed such as C(2 2 0) and C(1 1 1) which overlaps with the (0 0 2) line of the hexagonal phase and the line C(3 1 1) which also overlaps with the H(1 1 2) of the hexagonal phase.
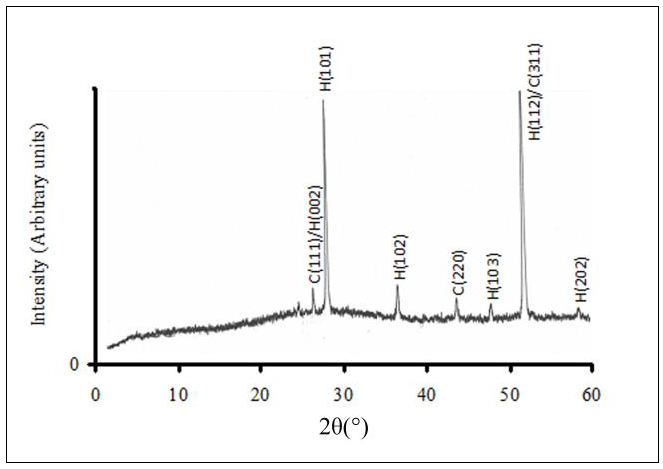 | Figure 1. XRD diffractogram of one of the CdS:In thin films prepared by the SP technique |
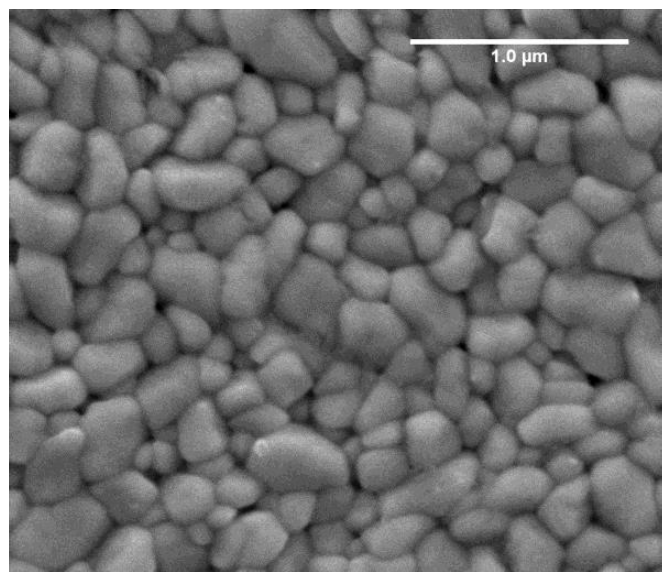 | Figure 2. SEM image of one of the CdS:In thin films prepared by the SP technique |
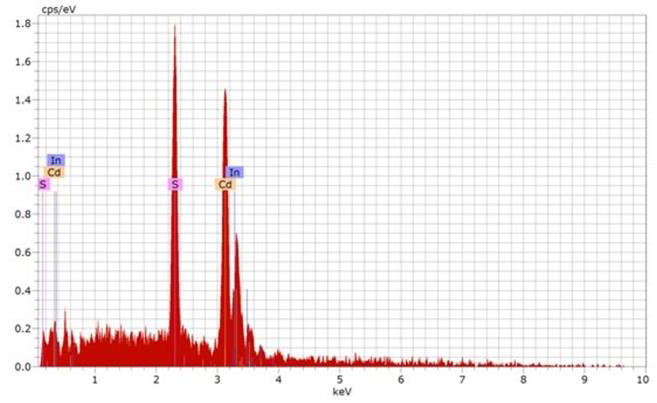 | Figure 3. EDS spectrum of one of the CdS:In thin films prepared by the SP technique |
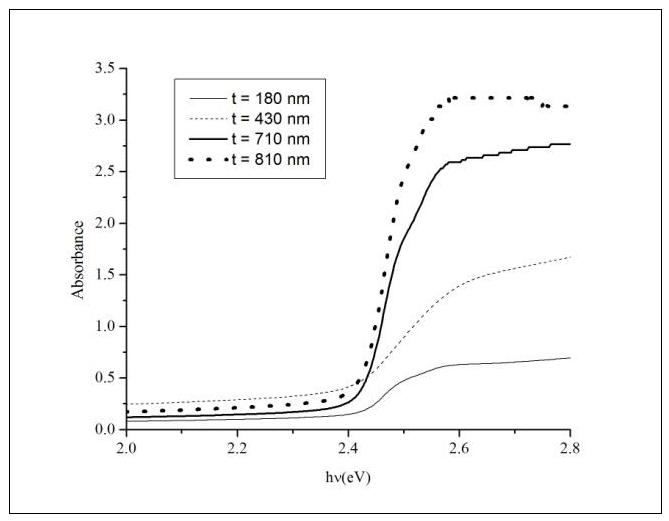 | Figure 4. Absorbance of CdS:In thin films of different thickness against the photon's energy (hν) |
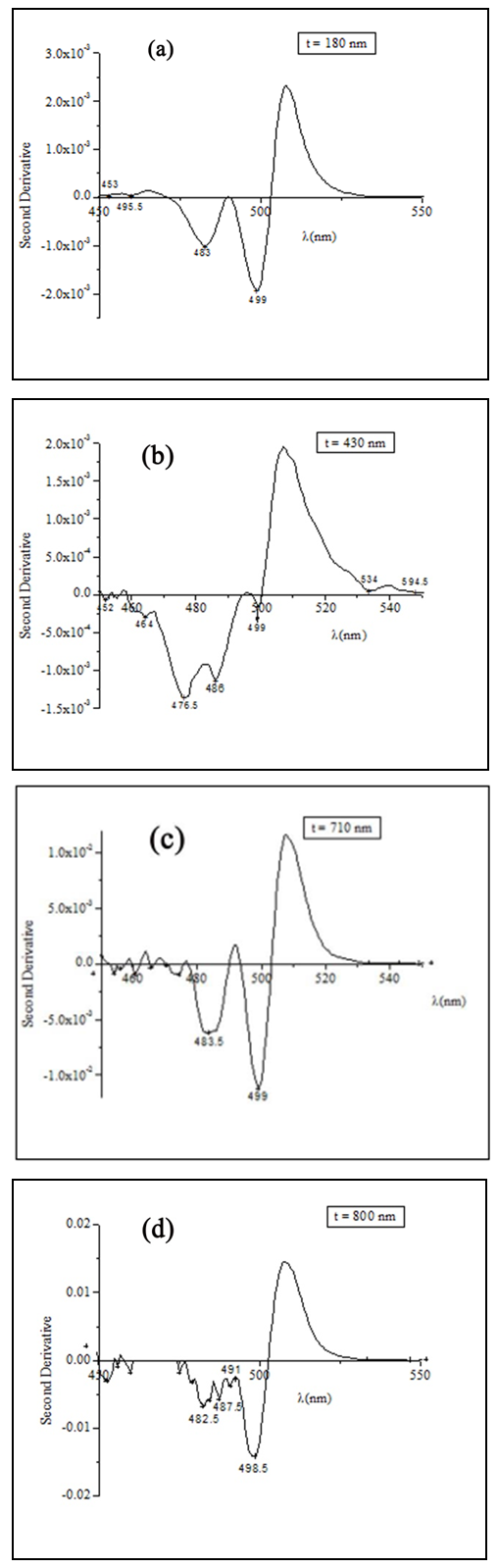 | Figure 5. The second derivative of CdS:In thin films of different thickness |
4. Conclusions
- Indium doped cadmium sulphide thin films were produced on glass substrates by the spray pyrolysis technique. XRD diffractogram showed that the films have predominantly wurtzite structure. SEM micrographs showed that that the films are polycrystalline with grains of deformed hexagonal and cubic shapes. EDS report showed that the films are cadmium rich and contain indium. The second derivative of the absorbance was used to detect the peaks in the absorption spectrum. The merged A and B free exciton peak and C free exciton peak of the hexagonal phase were observed and their energies were deduced and compared with those obtained by different authors. The separation between the merged A and B free exciton peaks and that of the C free exciton peak was calculated and found to be in good agreement with that of the bulk and with that obtained by different authors. The bandgap energy of the zinc-blined was obtained and found to be in good agreement with that in the literature.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML