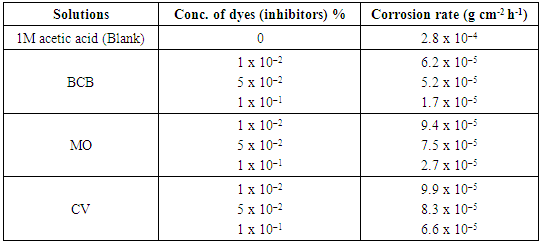-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2017; 7(3): 63-72
doi:10.5923/j.ijmc.20170703.03

Corrosion Behavior of Pure Lead in 1M Acetic Acid and Using Some Dyes as Corrosion Inhibitors
Azza El-Sayed El-Shenawy
Chemistry Department, Faculty of Science, Al-Azhar University, Cairo, Egypt
Correspondence to: Azza El-Sayed El-Shenawy, Chemistry Department, Faculty of Science, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

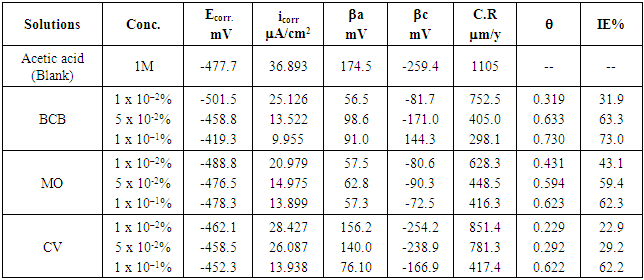
The corrosion behavior of lead in different concentrations of acetic acid (5 x10–2 – 1M) has been investigated. The effect of some dyes on the corrosion behavior of lead in 1 M acetic acid was investigated by means of weight loss measurements, open-circuit potential measurements and potentiodynamic polarization measurements. Brilliant cresyl blue (BCB), Mordant orange 37 (MO) and Crystal violet (CV) are used as effective corrosion inhibitors. The inhibition efficiency was increased with increase in inhibitor concentration. Polarization measurements reveal that, BCB has maximum IE value of 73% at 1x10-1 %. The results obtained from all the techniques are in good agreement and the values of inhibition efficiency are dependent upon the concentration and chemical structure of dyes.
Keywords: Pb, Acetic acid, Dyes
Cite this paper: Azza El-Sayed El-Shenawy, Corrosion Behavior of Pure Lead in 1M Acetic Acid and Using Some Dyes as Corrosion Inhibitors, International Journal of Materials and Chemistry, Vol. 7 No. 3, 2017, pp. 63-72. doi: 10.5923/j.ijmc.20170703.03.
Article Outline
1. Introduction
- Lead was one of the first used metals, due to its low melting point [1]. The soft, malleable and ductile metal was also used by the ancient Egyptians for the construction of statues and pipelines, but also for ceramic glazes, sinkers in fishing nets and ornaments [2]. Related products as lead carbonate and lead oxide were used as pigments in face powder, rouges and mascara [3]. Lead is frequently used in chemical plants and laboratories for fume ducts, hoods, bench tops and floors [4]. Lead is also used in buildings for roofing, gutters and downs pouts [5]. The metal was also alloyed with other elements such as tin for the production of pilgrim medallions, communion tokens and coins [6]. It is clear that, lead had many important applications due to the limited corrosion rate of it and its alloys. Acetic acid and acetate rapidly attack lead, even the protective film of lead oxide is very soluble in both acetic acid and acetate [7]. Organic acids belong to the group of the most important chemicals used in several industries, such as textile, leather, chemical, food, pulp, paper, drugs, plastics, petrochemicals. These acids are used as reagents for the manufacture of various chemicals ranging from drugs and pharmaceuticals to plastics [8-10]. Acetic acid is more frequently used as reactants or solvents in many industrial processes [11]. Some studies [12, 13] show the corrosion behavior of Al and stainless steel in acetic acid solutions. Azo compounds are the most widely used due to their versatile applications. Dyes possess molecular structures which recommend them for investigation as possible corrosion inhibitors. The inhibition of the corrosion by these dyes is mainly attributed to the formation of complex compounds between the metal-ions and the nitrogen of the azo binding at the electrode surface [14, 15]. The adsorption process depends on the electronic characteristic of the molecules, the chemical composition of the solution, nature of the metal surface, temperature of the reaction and on the electrochemical potential at the metal solution interface [16]. The adsorption occurs from active centers such as P, Se, S, N and O atoms, the double or triple bonds and also aromatic rings [17-20].Polyethylene glycol as a corrosion inhibitor for lead and lead free solders in acidic medium has been investigated [21] and the effect of different amino acids on the corrosion of lead in aqueous solution at different pH has been investigated [22].The objective of the present work is to study the effect of different concentrations of acetic acid on the corrosion behavior of lead and study the effect of some dyes on the corrosion of lead in 1M acetic acid solution.
2. Experimental Details
2.1. Materials and Reagents
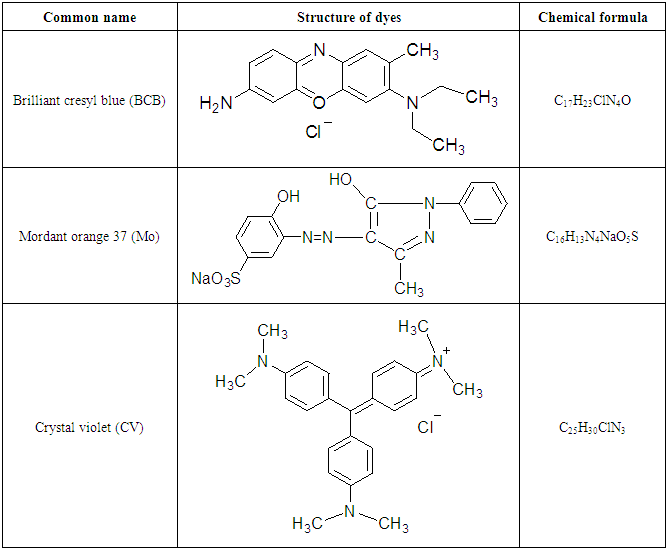
- The working electrode were made from spectroscopically pure lead 99.99% (Alderich-Chemic). The circular electrode of surface area of 1 cm2 was used in all techniques. The lead samples were polishes using different grades of emery paper (800-1000), washed with de-ionized water and dried at room temperature. AR grade acetic acid was used for all techniques. De-ionized water was used to prepare different conc. of acetic acid (5 x 10–2 –1 M). Different dyes were used as corrosion inhibitors, such as Brilliant cresyl blue (BCB), Mordant orange 37 (MO) and Crystal violet (CV), concentration range of dyes (1 x 10–2 % – 1 x 10–1 %). The structure and chemical formula of the dyes are shown in Table (1). The polarization experiments were carried out in a three – necked Pyrex glass assembly with a working electrode, platinum electrode as counter electrode and SCE (saturated calomel electrode) as reference electrode. All measurements were performed in freshly prepared and aerated solutions are room temperature (25 ± 1°C). Gravimetric experiments (weight loss method).
|
2.2. Chemical and Electrochemical Techniques
2.2.1. Weight Loss Measurements
- Measurements were carried out in glass beaker containing 50 cm3 of corrosive solution and corrosive solution with different concentrations of dyes. After an immersion time of 24 hours, the electrode was taken out and washed well with distilled water, dried and weighted accurately using digital balance (Analytical Balance Model FA 2104 A).
2.2.2. Open–circuit Potential Measurements and Potentiodynamic Polarization Measurements
- For open-circuit potential and polarization measurements, were performed on computerized potentiostat (Radiometer model volta Lab 40) and Volta master 4 soft ware. The polarization measurements were carried out and the anodic /cathodic polarization curves were recorded under a constant sweep rate of 5 mV/s, from -1500 to 1500 mV (SCE). The corrosion potential (Ecorr.) and corrosion current density (icorr.) were calculated using computer program method (Volta Lab Master 4).
3. Results and Discussion
3.1. Weight Loss Measurements
- The weight loss study of pure lead in 1M acetic acid and with present of different concentrations of dyes (1 x 10–2 –1 x 10–1 %) have been performed at room temperature. The corrosion rate of pure lead in 1M acetic acid in presence of different dyes as a function of concentration of dyes have been plotted in Fig. 1. It was observed from the nature of the curves in the figure, that there is a continuous decrease in the corrosion rate with increase in the amount of dyes in the solutions.
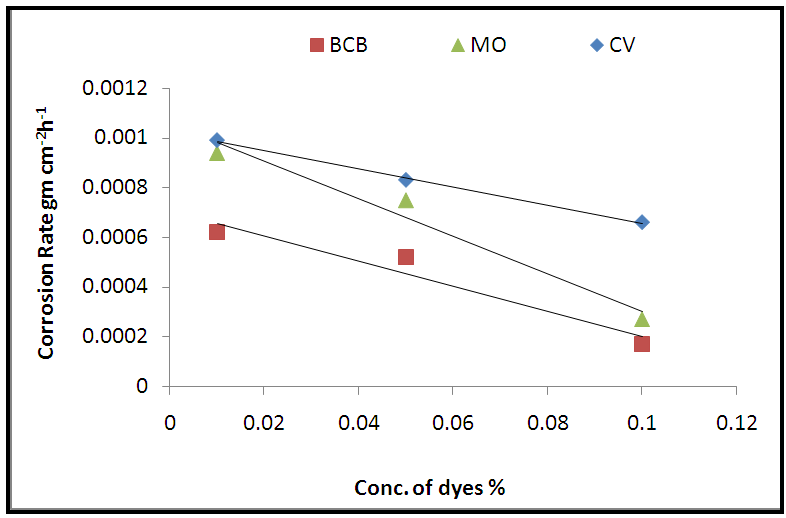 | Figure (1). Variation of corrosion rate with the concentrations of dyes at room temperature |
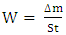 | (1) |
|
3.2. Open-Circuit Potential Measurements
3.2.1. Corrosion Behavior of Pure Lead in Inhibitor Free Acetic Acid Solution
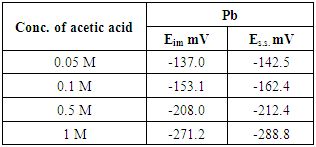
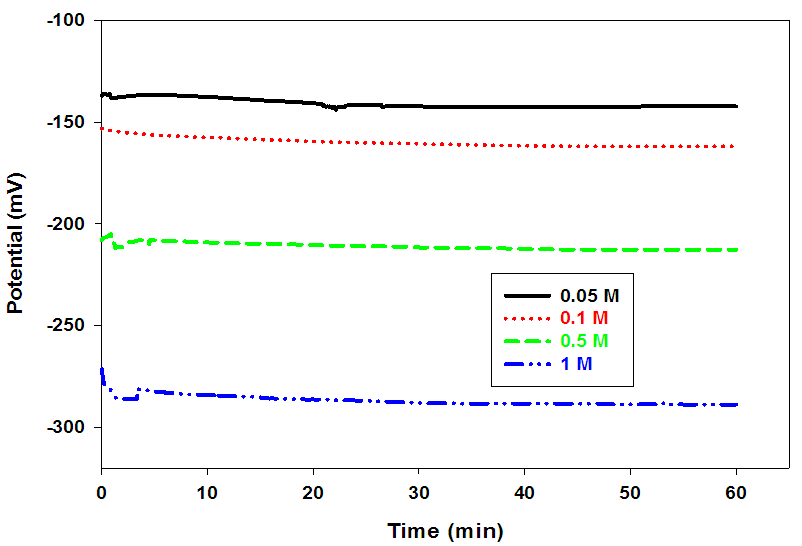
- The open circuit potentials of pure lead in the absence of dyes (inhibitors), in different concentrations of acetic acid (5 x 10–2–1M) were traced over 60 min from the electrode immersion in different concentrations of acetic acid. Fig. (2), illustrate the variation of the open-circuit potential for pure lead electrode with the time at room temperature. The values of Eim and Es.s for pure lead in different concentrations of acetic acid are summarized in Table (3).
 | Figure (2). Variation of the open circuit potential of Pb in different concentrations of acetic acid solution, at room temperature |
|
3.2.2. Corrosion Behavior of Pure Lead in 1M Acetic acid in Presence of Different Dyes (as inhibitors)
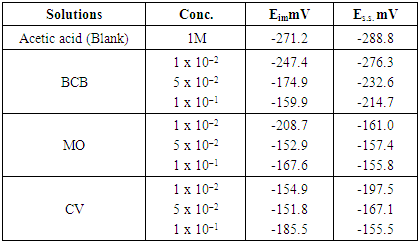
- Figs (3-5), illustrate the open-circuit potentials of Pb in the absence and presence of different dyes at constant concentration (1M) of acetic acid. The open-circuit potentials measurements were traced over 60 min. from the electrode immersion in the solution. The steady state potential is reached within the first 20 min. of the electrode immersion in the test solution.
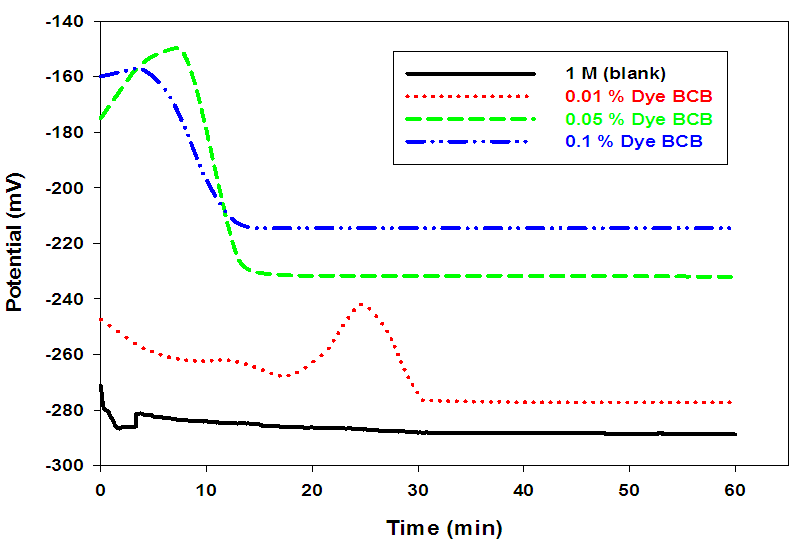 | Figure (3). Variation of the open circuit potential for Pb in 1 M acetic acid solution in the absence and presence of different concentrations of Dye BCB at room temperature |
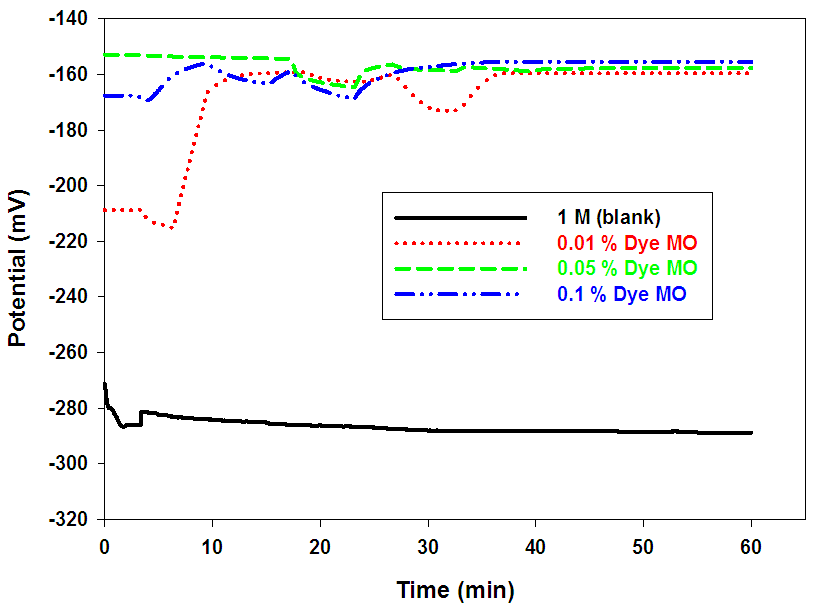 | Figure (4). Variation of the open circuit potential for Pb in 1 M acetic acid solution in the absence and presence of different concentrations of Dye MO at room temperature |
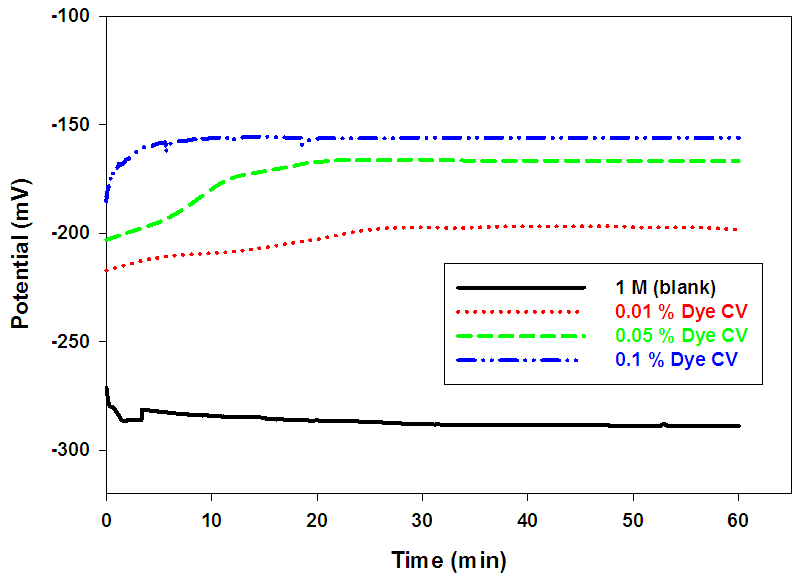 | Figure (5). Variation of the open circuit potential for Pb in 1 M acetic acid solution in the absence and presence of different concentrations of Dye CV at room temperature |
|
3.3. Potentiodynamic Polarization Measurements
3.3.1. Electrochemical Behavior in Different Concentrations of Acetic Acid
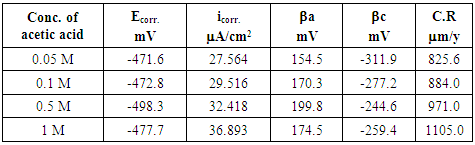
- Fig. 6 shows typical potentiodynamic curves of pure lead in various molar concentrations (0.05 - 1M) of acetic acid solutions from -1500 to 1500 mV(SCE) at a scan rate 5 mV/S. Inspections of the data (Table 5), reveal that, icorr. increases with increases in the molar concentration of the solution. The lead anode dissolves readily in acetic acid solution [24]. The dissolution rate increases with increases in the electrolyte concentration [24].
|
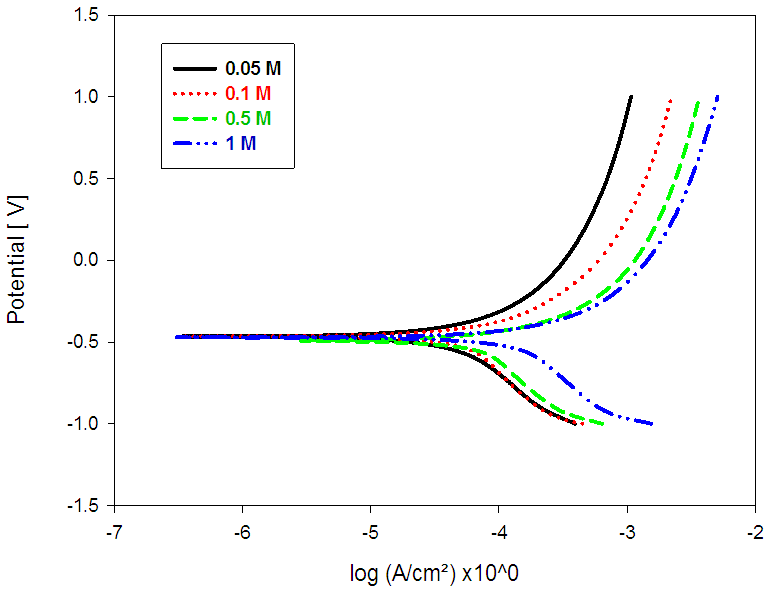 | Figure (6). Anodic and cathodic potentiodynamic polarization curves for Pb in different concentrations of acetic acid solution at room temperature |
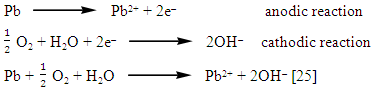
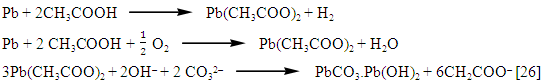 The precipitation of the salt layer (insoluble salt such as hydrocerussite 2PbCO3.Pb(OH)2 ) [27] on the anode surface, blocks the active surface sites and causes in activation of a part of the surface with respect to the corrosive medium [24].
The precipitation of the salt layer (insoluble salt such as hydrocerussite 2PbCO3.Pb(OH)2 ) [27] on the anode surface, blocks the active surface sites and causes in activation of a part of the surface with respect to the corrosive medium [24].3.3.2. Electrochemical Behavior of Pure Lead in Presence and Absence of Different Concentrations of Different Dyes
- Figs. (7-9), illustrate anodic and cathodic polarization cureves n the absence and presence of different concentrations of BCB, MO and CV dyes, as corrosion inhibitors for pure lead in 1M acetic acid solution. Corrosion potential (Ecorr.), corrosion current density (icorr.), cathodic and anodic Tafel slopes (βc and βa) were calculated from the polarization curves have been recorded in Table (6).
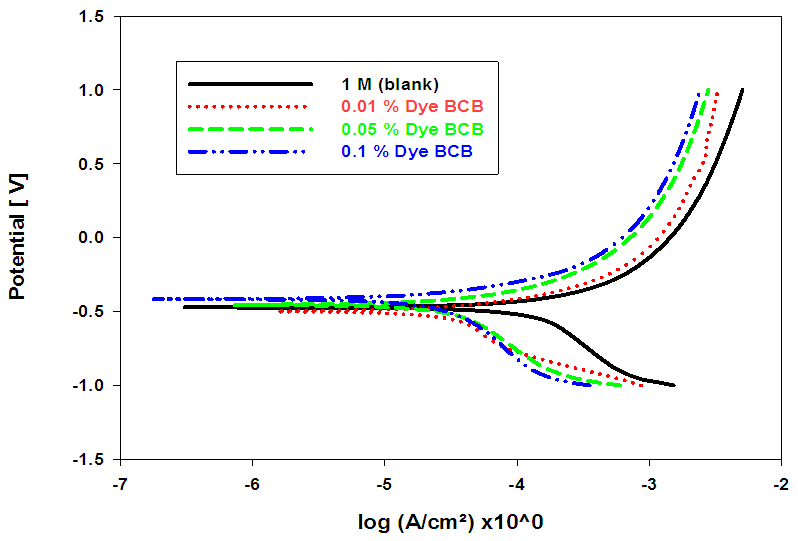 | Figure (7). Anodic and cathodic potentiodynamic polarization curves for Pb in 1 M acetic acid solution in the absence and presence of different concentrations of BCB Dye at room temperature |
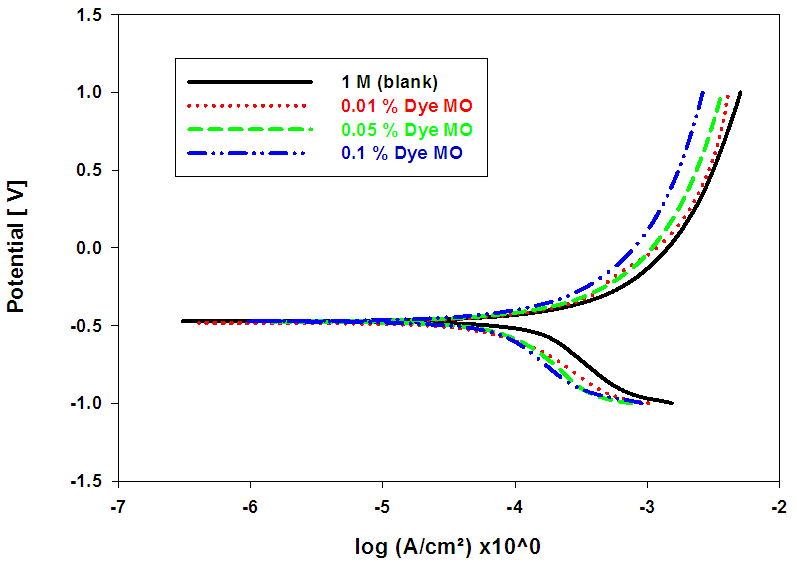 | Figure (8). Anodic and cathodic potentiodynamic polarization curves for Pb in 1 M acetic acid solution in the absence and presence of different concentrations of MO Dye at room temperature |
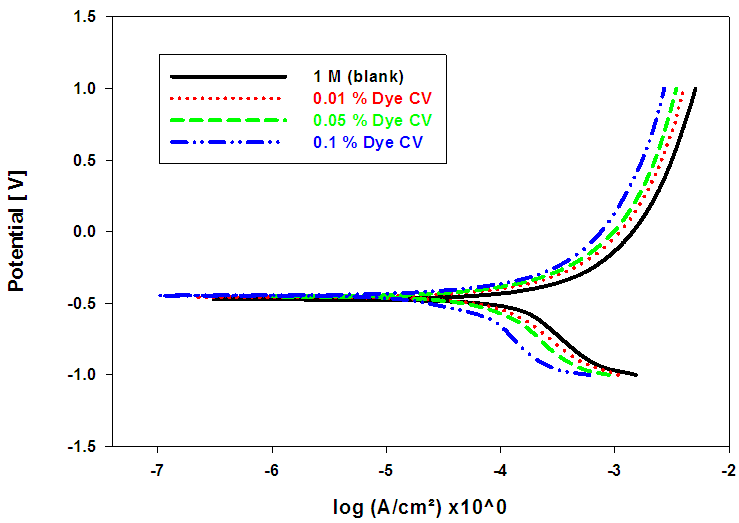 | Figure (9). Anodic and cathodic potentiodynamic polarization curves for Pb in 1 M acetic acid solution in the absence and presence of different concentrations of CV Dye at room temperature |
|
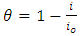 | (2) |
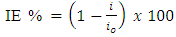 | (3) |
4. Conclusions
- 1- The investigated dyes are good inhibitors for pure lead surface in 1 M acetic acid solution.2- All dyes can be classified as mixed type inhibitors.3- The inhibitive effect of these dyes is attributed to the formation of good adsorption film on lead surface.4- The lead corrosion inhibition efficiency of the investigation dyes in 1M acetic follows the order: BCB dye> MO dye > CV dye
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML