-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2017; 7(3): 55-62
doi:10.5923/j.ijmc.20170703.02

Study of a Moroccan Clay Siltstone: Physicochemical Characterization and Approach of Their Sorption Properties Using Methylene Blue
O. Ait Malek1, S. Fakhi2, H. El Hadi1, S. El Aouidi3, A. Laissaoui3, A. Ayach2, A. Bouih3, M. Benmansour3, H. Yaakoubi4, S. Fait5
1Hassan II University of Casablanca, Geodynamics of Old Chains Laboratory, Faculty of Sciences Ben M'Sik Casablanca, Morocco
2Hassan II University of Casablanca, Engineering and Materials Laboratory (LIMAT), Thermostructural Materials, Polymers and Radiochemistry Team (TMPR), Faculty of Sciences Ben M'Sik Casablanca, Morocco
3National Energy Center of Sciences, and Nuclear Techniques (CNESTEN), Center for Nuclear Studies Maamoura (CENM), Salé, Morocco
4Hassan II University of Casablanca, Dynamic of Sedimentology Basin and Geologic Correlation Laboratory, Faculty of Sciences Ben M'Sik Casablanca, Morocco
5Hassan II University of Casablanca, Ecology and Environment Laboratory, Faculty of Sciences Ben M'Sik Casablanca, Morocco
Correspondence to: O. Ait Malek, Hassan II University of Casablanca, Geodynamics of Old Chains Laboratory, Faculty of Sciences Ben M'Sik Casablanca, Morocco.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Natural argillaceous siltstone was sampled from Ourika High Atlas of Marrakech, Morocco, and its application for methylene blue (MB) adsorption from aqueous solution was investigated. Physico-chemical characterization of the natural argillaceous siltstone was determined by X-ray Diffraction, fluorescence X, Scanning Electron Microscopy (SEM), laser diffraction to define particle size distribution and total organic matter content (OM) by Walkley and Black titration method. Batch adsorption studies revealed that MB adsorption on natural argillaceous siltstone decreased with increasing adsorbent amount and also increased with increase in initial solution pH with BM concentration. The adsorption process depends on initial MB concentration and attains equilibrium within 180 min. It was found that the Langmuir equation fit better than the Freundlich and Temkin equation. The adsorption kinetics of methylene blue is described by the pseudo second order reaction model. The obtained results are: (a) high levels of color removal (91%) were achieved with low contact times adsorbent/dye (less than 180 min contact); and (b) natural argillaceous siltstone can be successfully used as adsorbent of methylene blue in aqueous solutions. Natural argillaceous siltstone can be an alternative for more costly adsorbents used for dye removal in waste water treatment processes.
Keywords: Adsorption, Natural argillaceous siltstone, Methylene bleu, Environment
Cite this paper: O. Ait Malek, S. Fakhi, H. El Hadi, S. El Aouidi, A. Laissaoui, A. Ayach, A. Bouih, M. Benmansour, H. Yaakoubi, S. Fait, Study of a Moroccan Clay Siltstone: Physicochemical Characterization and Approach of Their Sorption Properties Using Methylene Blue, International Journal of Materials and Chemistry, Vol. 7 No. 3, 2017, pp. 55-62. doi: 10.5923/j.ijmc.20170703.02.
Article Outline
1. Introduction
- Within the global framework of environment protection and the reduction of pollutant migration, through their containment and storage as waste [1, 2], several previous works focused on the ability of natural geological materials and the role of their physico-chemical properties in trapping polluting elements and limiting their transport in the biosphere and geosphere [3-7]. Among many materials characterized and tested, it is appropriate to quote clays [8, 9], phosphates [10] and coal [11, 5, 12, 13].The aim of the present work is to investigate for local and low coast materials that can be used as a containment matrix for national waste. After testing the black shales [4], we are interested in argillaceous siltstones from Triassic age sandstone series located in Ourika, High Atlas of Marrakech area, Morocco.Before initiating the study of the gross rock physicochemical retention properties, we proceeded in the first place to define its chemical compound, mineralogical characterization, morphological structure and its particle size distribution.Subsequently, the sorption physicochemical properties of the material have been tested by methylene blue (MB), an adsorbate commonly used for this kind of study [12, 14]. Gross rock characterization was accomplished by X-rays diffraction, particle size distribution by laser diffraction and the morphological structure was by scanning electron microscope. Major elements content was measured by X‐Ray fluorescence.The study of sorption mechanisms of MB onto an argillaceous siltstone (GI2) has been undertaking by quantifying the amount of adsorbed MB as a function of initial pH, adsorbent mass, initial MB concentration and adsorbent/adsorbate contact time.
2. Experimental and Analytical Methods
2.1. Nature of the Adsorbent: Natural Argillaceous Siltstone
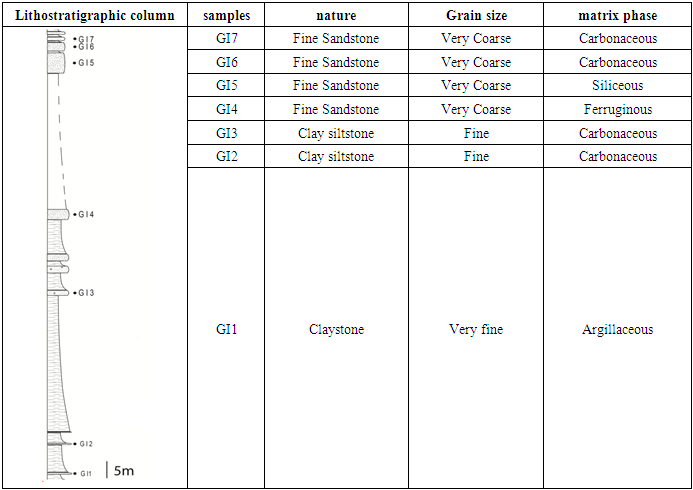
- Argillaceous siltstones are continental detrital rocks [15, 16]. Very abundant in Morocco, this kind of rock is primarily composed of quartz, feldspar, clay, mica and iron oxides [17]. Previous works on similar rocks have shown that they present an important sorption capacity of pollutant elements [18, 19]. The studied argillaceous siltstone rocks was chosen from a set of samples collected from Triassic age sandstone series located in Ourika, High Atlas of Marrakech area Morocco (Fig. 1). The analyzed powders are obtained after crushing and grinding the gross rock. In table (1) we gathered the different samples collected from the studied area and their macroscopic features determined using a polarization microscopy available in our laboratory.
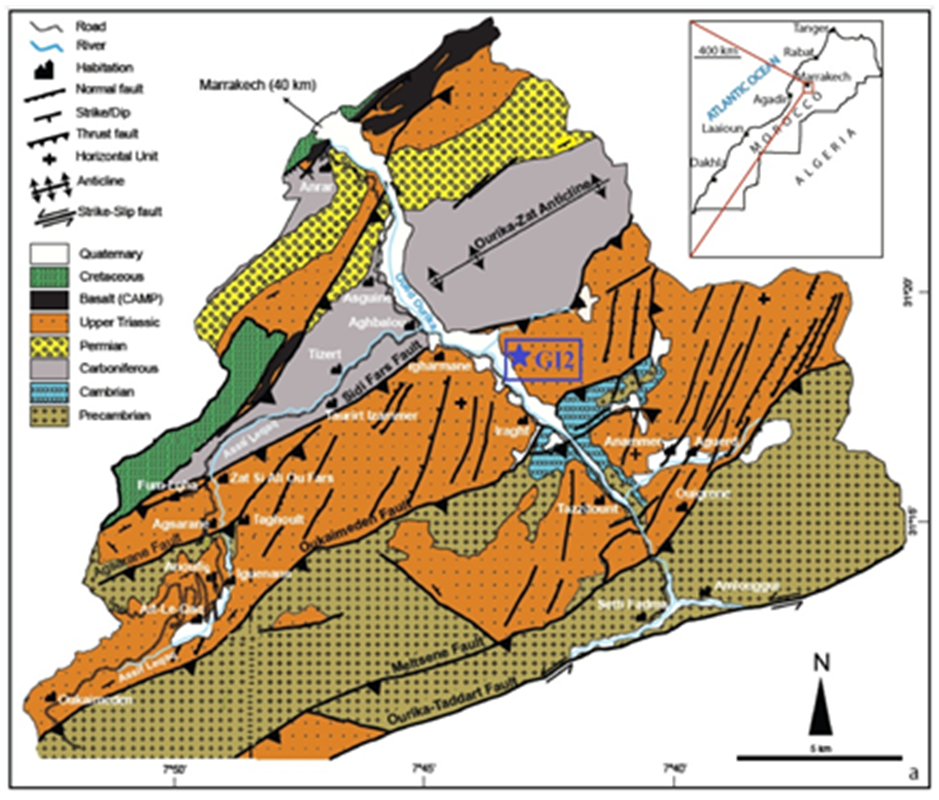 | Figure 1. Studied area location in the geological map of the central High Atlas basin [20], modified); GI: Ighermane |
|
2.2. Characterization Technics
2.2.1. Particle Size Distribution
- Particle size distribution of the homogenized sample was studied using an equipment of laser diffraction based on wet dispersion process (Malvern Mastersizer 2000), using a dispersion unit of Hydro 2000G. Particles distribution ranges between 0.02 and 2000 μm. A small amount of sample (≈ 1 g) was introduced into dispersion unit containing demineralized water, used as a dispersant, then measured after 10s.
2.2.2. X-ray Diffraction
- Mineralogical composition was identified by a diffractometer with XPERT-PRO System and a back monochromator in graphite, operating under 45 kV voltages and 40 mA intensity, with CuKα radiation as a source.
2.2.3. Organic Matter Content
- Organic matter content was determined by the Walkley and Black titration method [21] in the Geology Department laboratory of the Faculty of Sciences Ben M'Sik.
2.2.4. Scanning Electron Microscope (SEM)
- SEM analysis was carried out at the materials Platform of UATRS, CNRST of Rabat, Morocco. It was used to study and view the morphological surface of the samples grain.
2.2.5. X-ray Fluorescence
- Major elements compound was determined using an Axios X-ray fluorescence spectrometer with 1 kW wave-length dispersion, at the center UATRS of CNRST Rabat, Morocco.
2.3. MB Adsorption on Natural Argillaceous Siltstone
2.3.1. Theoretical Aspects
- Methylene blue adsorption on natural rocks was studied by several authors [22, 10, 8]. The kinetic adsorption modeling was carried out according to the pseudo first order, pseudo-second order and the intra-particles diffusion Kinetics, described successively by Lagergren [23] equation (1), Ho and McKay [24] equation (2) and Weber and Morris [25] equation (3).
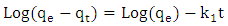 | (1) |
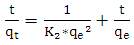 | (2) |
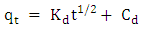 | (3) |
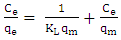 | (4) |
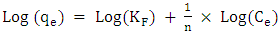 | (5) |
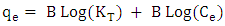 | (6) |
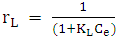 | (7) |
2.3.2. Tests of MB Sorption on Argillaceous Siltstone (GI2)
- The used compound in the present work is a pure methylene blue, with 319.86 g.mol-1 molar weight, supplied by Solvachim. Solutions were prepared by dissolving a required quantity of the dye in distilled water. The predefined pH value of methylene blue solutions was around 5.3. The agitation speed was fixed at 300 rounds per minute and the temperature was maintained ambient in all experiments. The effect of adsorbent mass was determined out by contacting three argillaceous siltstones mass (0.5 g; 1 g and 1.5 g) in a volume of 50 mL with 27.52 mg.L-1 methylene blue concentration. The effect of the solution pH was investigated by contacting during 2h, 50 mL of each methylene blue solution at an initial concentration of 26.06 mg.L-1 with 0.5 g of argillaceous siltstone. Solutions pH were adjusted in a range of 3.07 to 12 using HCl (1%) and NaOH (0.1 mol/L) dilute solutions.The solution concentration effect was performed by contacting 50 mL of each solution at various initial MB concentrations (16.09; 27.52; 36.37; 47.31; 59.17 and 82.8 mg.l-1) with 0,5g of natural argillaceous siltstone (GI2), the initial solutions pH was maintained (5.3).The residual MB concentration in the supernatant solutions was analyzed using an equipment of UV spectrophotometer (UV-1600PC), by monitoring absorption changes at a maximum absorption wavelength (665 nm).The data obtained was used to calculate methylene blue adsorbed amount at equilibrium, using the following expression [6, 27]:
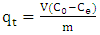 | (8) |
3. Results and Discussion
3.1. Characterization of the Argillaceous Siltstone (GI2)
3.1.1. Particle Size Distribution
- Particle size distribution of the adsorbent (table. 2) shows that the gross rock (GI2) contained 71% silt, 26% clay and 3% sand. This result gives our sample an argillaceous silt character.
|
3.1.2. X-ray Diffraction
- X-ray spectrum analysis of the adsorbent illustrated in figure (2), revealed the presence of an intense peak identified as quartz, with other peaks less intense of hematite, dolomite and muscovite, which implies that our adsorbent is heterogeneous. Clay was not detected by the device given its lower content. The organic matter content was 1.86%.
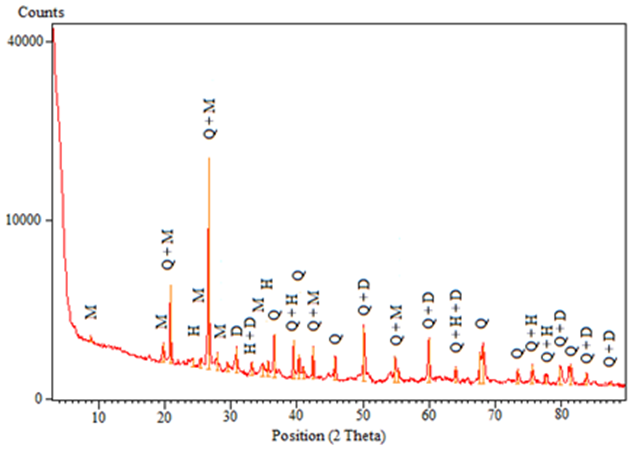 | Figure 2. Diffractogramme of studied argillaceous siltstone, Q: Quartz, M: Muscovite, D: Dolomite, H: Hematite |
3.1.3. Scanning Electron Microscope
- Scanning electron microscope analysis of the studied sample (Figure. 3) shows only the quartz grains with microcavities and micropores on the surface. Clay and dolomite particles are smaller and were not well visualized by the device.
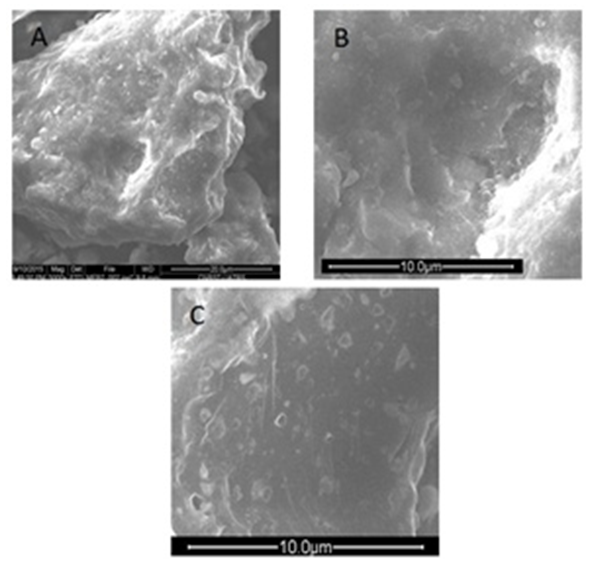 | Figure 3. SEM micrographs of argillaceous siltstone. A: angular quartz grain, B: cavity on quartz grain, C: micropores on quartz grains |
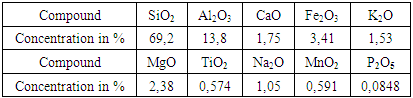
3.1.4. Major Elements Composition
- The major compounds of our sample (table. 3) evolve in descending order, according to the following sequence: SiO2> Al2O3> Fe2O3>MgO>CaO> K2O> Na2O> MnO2> TiO2> P2O5. Silica high content was assigned to the dilution of the sample by detrital quartz.
|
3.2. Adsorption Study
3.2.1. Adsorbent Mass Effect
- Adsorbent amount is an important parameter which determines the capacity of an adsorbent for a given initial concentration of the adsorbate. The experiments were repeated three times to eliminate uncertainties, and only the mean values were reported in figure (4). As seen in this figure, the adsorbed amounts of MB increase as the adsorbent mass decrease.
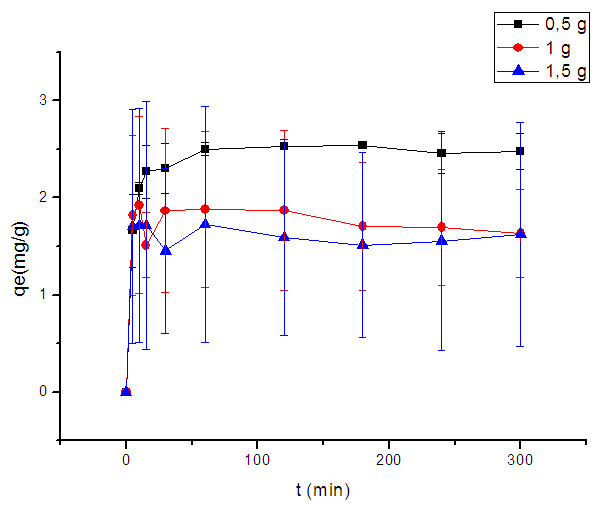 | Figure 4. Plot of adsorbent mass variation effect on MB adsorbed amount |
3.2.2. Effect of Initial Solution pH
- Figure (5) shows the effect of the initial solution pH on MB adsorption by argillaceous siltstone (GI2). From this figure, the adsorbed amount (qe) is maximum at pH 9.95. As well, the increase of the (qe) as a function of solution pH is in agreement with interior works [39, 40]. This can be explained by the fact that at low pH values, the adsorbent surface would be surrounded by H+ ions, which decrease the interaction of methylene blue ions with the adsorbent active sites, however, in a high pH, H+ ions concentration decreases which generates a good interaction between MB ions and the adsorbent surface cites [41]. The pH of the solutions of the other experiments was not adjusted (pH=5,3).
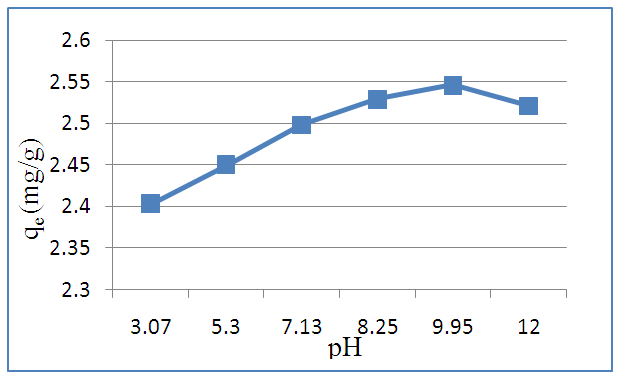 | Figure 5. Plot of initial solution pH Variation effect on MB adsorbed amount (qe) |
3.2.3. Contact Time and the Initial Concentration Effect
- The equilibrium adsorption capacity of the adsorbent for methylene blue increased with increasing initial dye concentration, as it is shown in figure (6). This figure shows that the equilibrium adsorption capacity increases from 2.6 to 5.1mg.g-1 with an increase in initial MB concentration from 27.52 to 82.8mg.L-1. These results may be due to the fact that increasing MB concentration induces an elevation of the driving force of concentration gradient, leading to an increase in MB molecules dissemination through the adsorbent surface [42, 43].
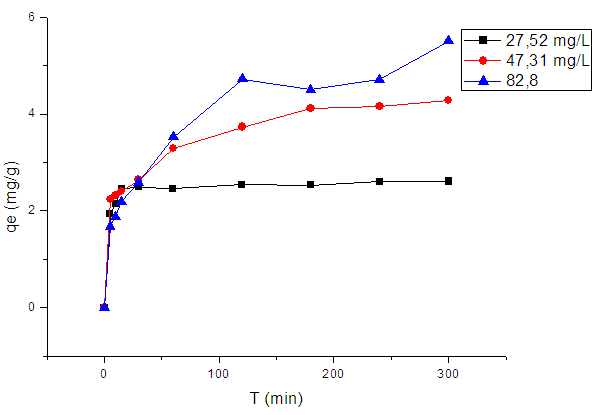 | Figure 6. Plot of adsorbed MB amount vs. contact time |
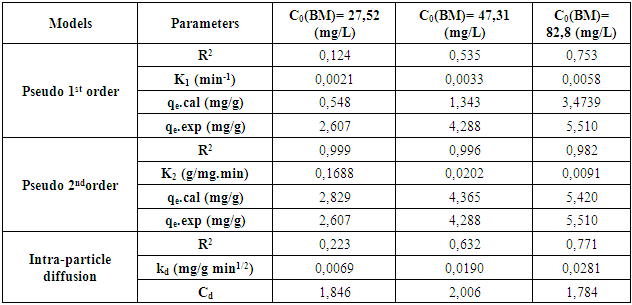
3.2.4. MB Adsorption Kinetic onto Argillaceous Siltstone
- The behavior of the MB adsorption process was analyzed by using the pseudo-first order, pseudo-second-order, and intraparticle diffusion models. The constants values, the adsorbed amount values and the correlation coefficients R2 values were calculated from the slopes of the respective linear plots of each model and are summarized in table (4). The best-fit model was selected based on the linear regression correlation coefficient, R2, values.
|
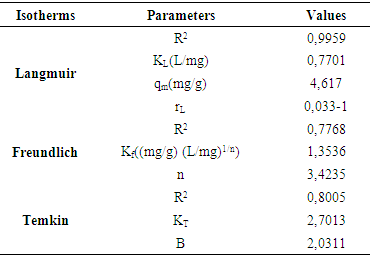
3.2.5. Adsorption Isotherms
- According to the work of Giles [45], the graphical representation of the adsorbed amount versus residual concentration at equilibrium, indicates the adsorption isotherm type. However, figure (7) indicates that the adequate isotherm is that of type L, suggesting that the adsorption of MB by argillaceous siltstone (GI2) process occurs in a monolayer.
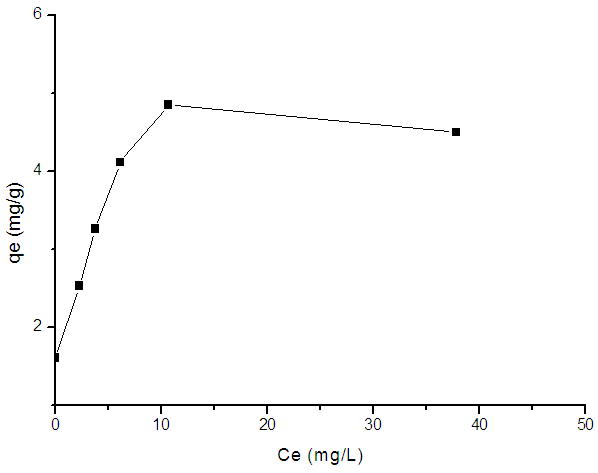 | Figure 7. Isotherm of MB adsorption onto argillaceous siltstone after Giles (1960) |
|
4. Conclusions
- The main aim of this work was to test a Moroccan argillaceous siltstone performance in the retention of organic and inorganic substances. The study of this rock and its application to eliminate methylene blue from aqueous solution allowed us to conclude that:ü The adsorbent is rich in silt with a non-negligible clay amount and a small amount of sand. ü The mineralogical compound revealed the presence of a large amount of quartz and a very low amount of hematite, dolomite and muscovite, indicating the heterogeneity of this material. ü Morphological analysis of the sample shows the presence of micro-cavities and micro-pores on the quartz surface. ü The low oxide levels such Fe2O3, MgO, CaO, Na2O, K2O and MnO2 is due to the high content in silica, which is assigned to the dilution of our sample by quartz.ü The adsorption rate increases as much as the adsorbent mass decreases to reach a maximum percentage removal of 94,73% for 0.5g adsorbent mass;ü The adsorbed MB amount increases with increase of solution pH;ü The kinetic study shows that equilibrium time depends on initial MB concentration. When the initial MB concentration increases the methylene blue adsorbed amount increases, while the percentage removal decreases. ü The adsorption kinetic is described by the Pseudo-second order model, suggesting that the MB adsorption on argillaceous siltstone (GI2) is done by chemisorption process;ü The adsorption isotherm is well described by Langmuir model which indicates that MB adsorption on argillaceous siltstone (GI2) is done in monolayer and the homogeneous distribution of active sites onto the surface of the studied argillaceous siltstone.
ACKNOWLEDGMENTS
- This work is performed in the framework of the Mixed Research Unit (UMR) between the National Energy Center of Sciences, and Nuclear Techniques (CNESTEN) and Hassan II University of Casablanca.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML