-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2017; 7(3): 47-54
doi:10.5923/j.ijmc.20170703.01

Evaluation of the Inhibitive Effect of Annona Muricata .L Leaves Extract on Low-Carbon Steel Corrosion in Acidic Media
N. B. Iroha1, M. A. Chidiebere2
1Department of Chemistry, Federal University Otuoke, Yenagoa, Nigeria
2Electrochemistry and Materials Science Research Laboratory, Department of Chemistry, Federal University of Technology, Owerri, Nigeria
Correspondence to: N. B. Iroha, Department of Chemistry, Federal University Otuoke, Yenagoa, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The inhibition of low-carbon steel corrosion in 1 M HCl by extracts of Annona Muricata .L leaveswas investigated using gravimetric and electrochemical techniques. The results show that theleaves extractfunctioned as effective corrosion inhibitor for low-carbon steel and its inhibition efficiency was concentration dependent. Weight loss method showed that the inhibition efficiency of the extracts increases with increase in its concentration and exposure time but decreases with increase in temperature. Potentiodynamic polarization results showed Annona Muricata .L leaves to be a mixed type inhibitor in 1 M HCl environment, whereas the electrochemical impedance spectroscopy results revealed adsorption of the extracts species on the steel surface. Fitting of the experimental data to the Arrhenius equation revealed that the constituents of the leaves extract were physically adsorbed on the corroding carbon steel surface. The experimental data complied with the Langmuir adsorption isotherm and the negative values of the Gibb’s free energy of adsorption obtained suggested that inhibitor molecules have been spontaneously adsorbed onto the carbon steel.
Keywords: Steel, Annona Muricata .L, Corrosion inhibitor, Weight loss, Potentiodynamic polarization, Electrochemical impedance spectroscopy
Cite this paper: N. B. Iroha, M. A. Chidiebere, Evaluation of the Inhibitive Effect of Annona Muricata .L Leaves Extract on Low-Carbon Steel Corrosion in Acidic Media, International Journal of Materials and Chemistry, Vol. 7 No. 3, 2017, pp. 47-54. doi: 10.5923/j.ijmc.20170703.01.
Article Outline
1. Introduction
- Iron and its alloys are very useful in diverse industrial and structural applications. Acid solutions like hydrochloric acid are widely used in the chemical industry for removal of undesired scales and rust, processes which usually leads to metal corrosion. Since aggressive acid solutions are widely used for industrial purposes, the addition of inhibitors effectively secures the metal against an acid attack.Most synthetic corrosion inhibitors are highly toxic to both human beings and the environment. This has motivated the use of some natural products as corrosion inhibitors. Most of the natural products are non-toxic, biodegradable and readily available [1-3]. Most researchers recently started studying the application of extracts of some common plant as corrosion inhibitors [4-9]. These extracts contain different hydroxy organic compounds, e.g. tannins, pectin, flavoniods, anthraquinones, steroids, saponins and coumarins in addition to other nitrogen containing compounds.The leaves of Annona muricata .L are dark green, glossy, oblong and smooth, with no hairs. The phytochemical screening of Annona muricata L leaves revealed the presence of flavonoids, saponins, terpenes, tannins and alkaloids [10]. The major constituent in the leaves of Annona muricata L is an alkaloid named 6-hydroxyundulatine among other alkaloids. The presence of these heterocyclic compounds is believed to be responsible for its inhibitory action.
2. Materials and Methods
2.1. Material Preparation
- Carbon steel coupons of dimension 2 x 4 x 0.15cm with weight percentage composition as follows: C, 0.05; Mn, 0.6; P, 0.36; Si, 0.3; and the balance Fe were used in this study. The coupons were prepared and cleaned as described elsewhere [9]. The blank corrodent was a solution of 1.0 M HCl.Stock solutions of Annona muricata .L leaves extract were prepared by boiling weighed amounts of the dried and ground plant material for 4 h in 1 M HCl. The solutions were cooled and then filtered and stored. From the respective stock solutions, inhibitor test solutions were prepared in the concentration range 10–50 v/v% using excess acid as solvent.
2.2. Gravimetric Experiments
- Gravimetric experiments were conducted under total immersion conditions maintained at 303, 313 and 323K. The pre-cleaned and weighed coupons were suspended in beakers containing the test solutions using a glass rod and hook. All tests were made in aerated solutions. To determine weight loss with respect to time, the coupons were retrieved from test solutions at 24 hours interval, appropriately cleaned, dried, and reweighed. The weight loss was taken to be the difference between the weight of the coupons at a given time and its initial weight. All tests were run in triplicate and the data showed good reproducibility. From the weight loss data, the surface coverage (θ) as a result of adsorption of inhibitor molecules, and inhibition efficiencies of the molecules (η%) were determined using equations (1) and (2), respectively.
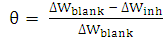 | (1) |
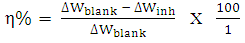 | (2) |
2.3. Electrochemical Measurements
- Electrochemical tests were conducted using a PAR-2273 Advanced Electrochemical System workstation, with a conventional three-electrode corrosion cell. A platinum sheet and a saturated calomel electrode (SCE) were used as a counter and reference electrodes, respectively. A metal specimen fixed in epoxy resin with a surface area of 1 cm2 exposed to the test solution, served as the working electrode. Electrochemical measurements were carried out in aerated and unstirred solutions at the end of 3600 s of immersion, which allowed the OCP values to attain steady state. Temperature was fixed at 30±1°C. Impedance measurements were performed at corrosion potentials (Ecorr) over a frequency range of 100 kHz - 0.1 Hz, with a signal amplitude perturbation of 5 mV. Potentiodynamic polarization studies were conducted in the potential range +250 to – 800 mV versus corrosion potential at a scan rate of 0.333 mV/s. Each test was run in triplicate to verify the reproducibility of the systems.
3. Results and Discussion
3.1. Gravimetric Experiments
- Figure 1 presents the effects of Annona Muricata .L leaves extract on the corrosion behavior of carbon steel in 1 M HCl solution at different temperatures studied. The results show that weight loss reduced in the presence of Annona Muricata .L leaves extract compared to the blank acid solution. It was also observed that weight loss of carbon steel in the presence of the inhibitor decreased with increase in concentration, indicating that the protection ability of the inhibitor is concentration dependent. From Figure 2, the inhibition efficiency increases with increase in the concentration of Annona Muricata .L leaves extract. This trend results from the increase adsorption of the phytoconstituents present in the inhibitor onto the carbon steel surface. The adsorption of these organic compounds on the metal surface probably creates a barrier for mass and charge transfers thus protecting the metal surface from corrosion. The degree of protection increases with the increasing surface coverage occupied by the adsorbed molecules.
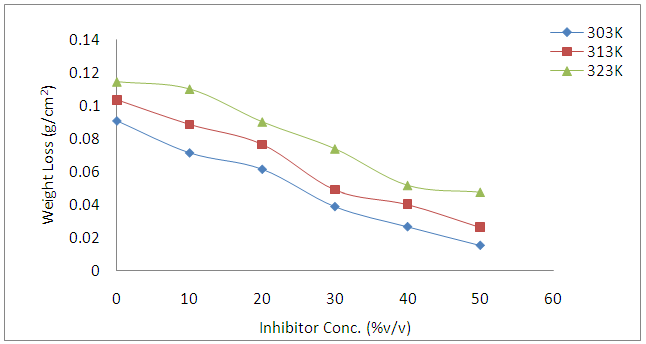 | Figure 1. Effect of Annona Muricata .L concentration on the weight loss of carbon steel in 1M HCl at different temperatures |
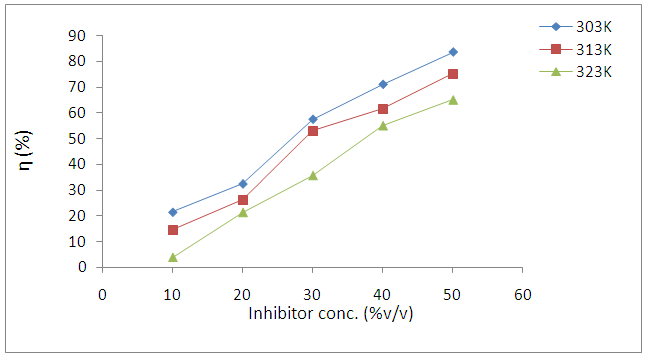 | Figure 2. Variation of η% with Inhibitor Concentration for Carbon steel in 1M HCl containing Annona Muricata .L at different temperatures |
3.2. Effect of Temperature
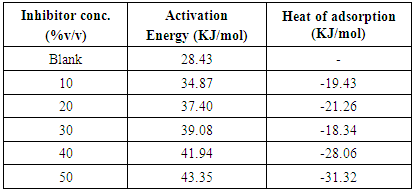
- The influence of temperature on the corrosion behaviour of carbon steel in 1M HCl in the absence and presence of Annona Muricata .L leaves extract were investigated by weight loss method at 303K, 313K and 323K. Corrosion rate is seen to increase with temperature rise both in the absence and in the presence of the additives. From Figure 2, it is observed that inhibition efficiency increases with increase in the concentration of Annona Muricata .L leaves extract but decreases with increase in temperature. Decrease in inhibition efficiency with increase in temperature is suggestive of physical adsorption of Annona Muricata .L leaves extract onto the carbon steel surface [11].Also, in examining the effect of temperature on the corrosion inhibition process, the apparent activation energies (Ea) were determined from the Arrhenius-type relationship between the corrosion rate (k) of carbon steel in acidic media and temperature (T) often expressed by the Arrhenius equation:
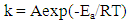 | (3) |
|
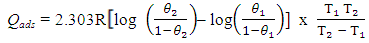 | (4) |
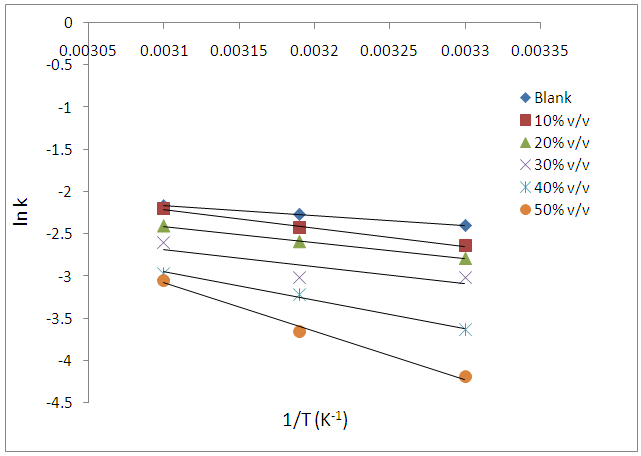 | Figure 3. Arrhenius plots for carbon steel corrosion in 1 M HCl without and with different concentrations of Annona Muricata .L leaves extract |
3.3. Potentiodynamic Polarization Consideration
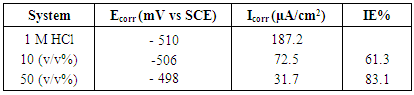
- The potentiodynamic polarization curves of carbon steel in the absence and presence of Annona Muricata .L are shown in Figure 4, for the sample in 1 M HCl solution. The values of the polarization parameters are provided in Table 2 where Ecorr and icorr are, respectively the corrosion potential and current density. Both were obtained from the extrapolation of the anodic and cathodic Tafel slopes with respect to the Ecorr values. The inhibition efficiency was calculated using the formula presented elsewhere [15]. The values in Table 2 reveal that in the presence of Annona Muricata .L and at higher concentration of 50 (v/v %) carbon steel displayed lower icorr and slightly more positive Ecorr values in studied environment. Therefore, one may conclude that Annona Muricata .L plays a major role in slowing down the evolution rate of hydrogen at the identified concentrations by forming a stable or insoluble film, which impede further dissolution, and the degree of inhibition depends on the concentration of the inhibitor. Corrosion decreases and IE% values increases by increasing the concentration of Annona Muricata .L. Therefore, it can be affirmed that inhibitors’ addition causes delay to carbon steel’s cathodic and anodic reactions in the tested solution. The transformation of the interface of the solution from active to passive dissolution state is due to the adsorption of Annona Muricata .L species on a corroding metal surface; thereby, developing a protective film.
|
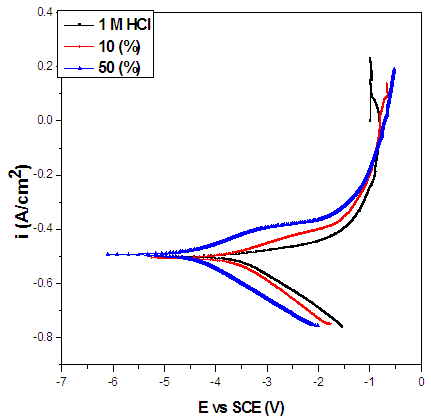 | Figure 4. Potentiodynamic polarization curves of carbon steel in 1 M HCl in the absence and presence Annona Muricata .L leaves extract |
3.4. Electrochemical Impedance Spectroscopy Considerations
- The Nyquist plots showed a capacitive loop followed by an inductive loop for the carbon steel samples in 1 M HCl solution as shown in Figure 5. The diameter of the semi circles are related to charge transfer resistance. The size of the capacitive loops was greater in the presence of Annona Muricata .L, compared to that in the absence of the inhibitor; an indication of a higher corrosion resistance for carbon steel in the presence of Annona Muricata .L. The occurrence of an inductive loop in both aggressive solutions may indicate certain non–faradaic processes, such as adsorption and desorption of corrosion products, occurring at the sample/electrolyte interface. The equivalent circuit model shown in Figure 6 was used to model the impedance results obtained for carbon steel in both solutions, after fitting with Zsimpwin software. The obtained results are presented in Table 3.
|
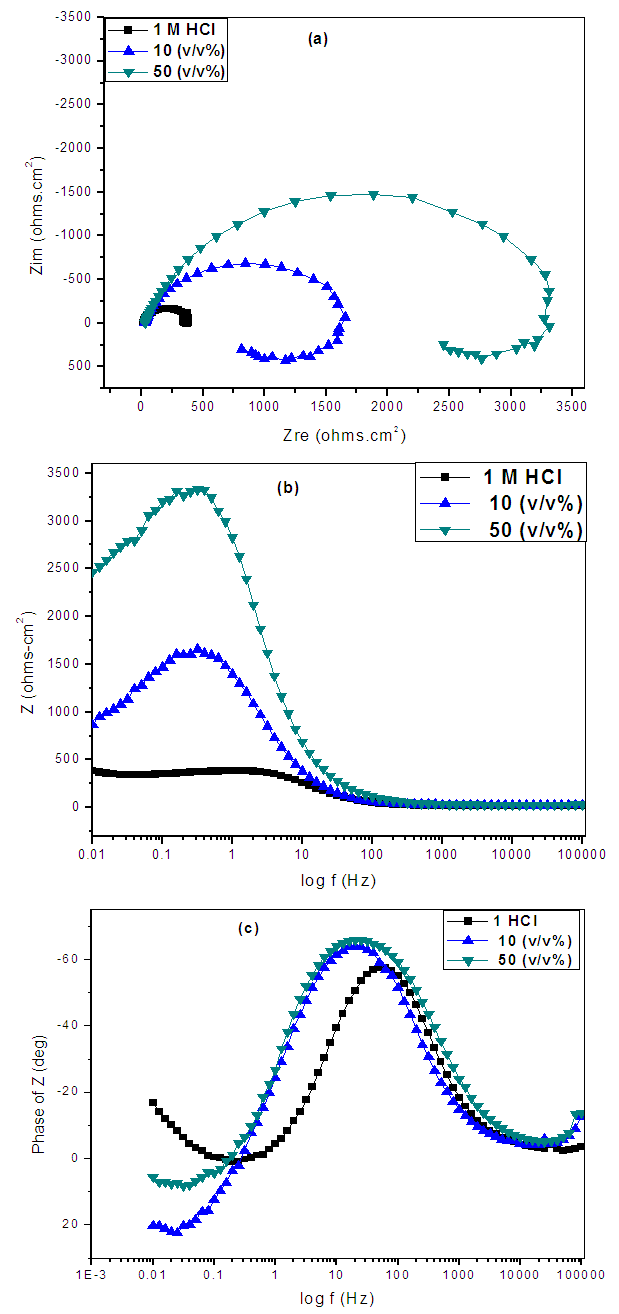 | Figure 5. Nyquist plots of the electrochemical impedance spectra on carbon steel in 1 M HCl in the presence and absence of Annona Muricata .L |
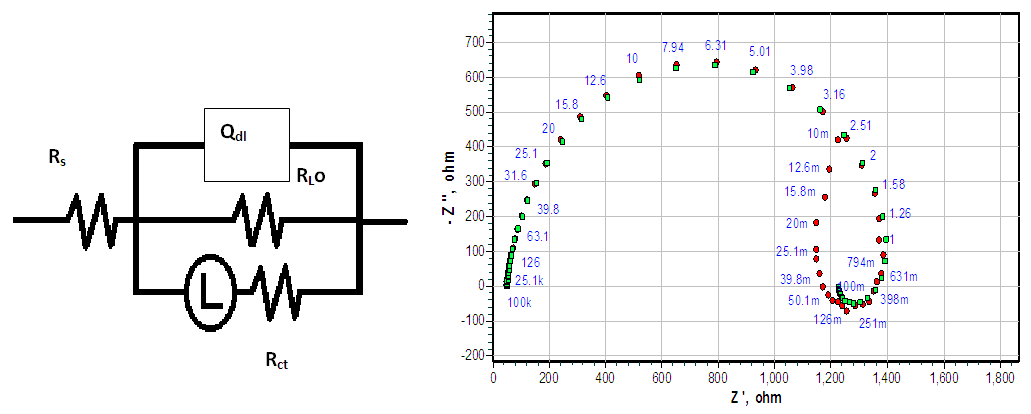 | Figure 6. Equivalent Circuit Model for carbon steel sample |
3.5. Adsorption Considerations
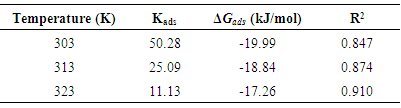
- The effectiveness of organic compounds as corrosion inhibitors can be ascribed to the adsorption of molecules of the inhibitors through their polar functions on the metal surface. Adsorption isotherm values are important to explain the mechanism of corrosion inhibition of organo-electrochemical reactions. The frequently used adsorption isotherm include: Langmuir, Frumkin, Hill de Boer, Parsons, Temkin, Flory-Huggins, among others. Langmuir isotherm was tested for its suitability to the experimental data. Langmuir isotherm is given by the expression:
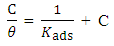 | (5) |
|
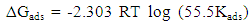 | (6) |
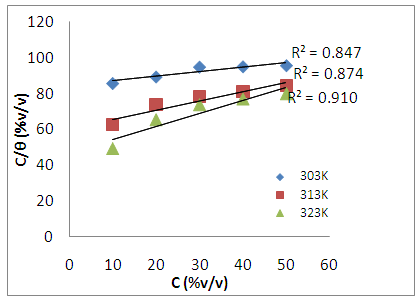 | Figure 7. Langmuir Isotherm for the adsorption of Annona Muricata .L leaves extract on carbon steel in 1M HCl at different temperatures |
4. Conclusions
- Annona Muricata .L leaves extract was found to be an efficient inhibitor for carbon steel in 1 M HCl solution. The rate of corrosion of the carbon steel in 1 M HCl is a function of the concentration of the inhibitor. This rate decreased as the concentration of the inhibitor is increased. The percentage inhibition efficiency of this inhibitor decreased as the temperature increases which indicate that physical adsorption was the predominant inhibition mechanism. Annona Muricata .L leaves extract is an eco-friendly corrosion inhibitor for carbon steel in 1 M HCl solution, so it can be used to replace toxic and expensive corrosion inhibitors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML