-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2017; 7(2): 25-35
doi:10.5923/j.ijmc.20170702.02

Comparative Sorption Studies of Dyes and Metal Ions by Ni/Al-Layered Double Hydroxide
Ayawei Nimibofa, Ebelegi N. Augustus, Wankasi, Donbebe
Department of Chemical Sciences, Niger Delta University, Wilberforce Island, Nigeria
Correspondence to: Ayawei Nimibofa, Department of Chemical Sciences, Niger Delta University, Wilberforce Island, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Anionic clay Ni/Al Layered double hydroxide (Ni/Al-LDHs) with M2+:M3+ of (4:1, 3:1, 2:1 and 1:1) ratios were synthesized by co-precipitation method from nitrate salt solutions. The synthesized LDHs were characterized by thermo-gravimetric analysis, Fourier transform infrared spectroscopy, X-Ray Diffraction, Energy Dispersive Spectroscopy/Scanning Electronic Microscopy and Thermogravimetric spectroscopy. The effects of contact time, temperature and initial concentrations on the adsorption of lead, copper, congo red and methylene blue were investigated to optimize the conditions for maximum adsorption. The experimental data from the batch adsorption studies were subjected to equilibrium studies using Langmuir, Freundlich, Temkin and Dubinin-Kaganer-Radushkevic isotherms and found to be consistent with physical adsorption. The results from the thermodynamic studies also indicated, that the adosprtion of dyes was endothermic while that of metal ions was exothermic.
Keywords: Kinetics, Adsorption, Thermodynamics, Methylene blue, Lead, Congo red, Copper, Equilibrium
Cite this paper: Ayawei Nimibofa, Ebelegi N. Augustus, Wankasi, Donbebe, Comparative Sorption Studies of Dyes and Metal Ions by Ni/Al-Layered Double Hydroxide, International Journal of Materials and Chemistry, Vol. 7 No. 2, 2017, pp. 25-35. doi: 10.5923/j.ijmc.20170702.02.
1. Introduction
- The industrial revolution of the past four decades has also caused serious pollution problems to mankind such that our continued existence on earth is threatened. Several pollutants such as dyes and heavy metals are continually released to aquifers and other water bodies all over the world. This has lead Governments and Scientists all over to scramble for solutions with a view to saving mother earth. Major sources of pollutants such as dyes and heavy metals includes petrochemical industries, textiles industries, mines, etc [1, 2]. They are known to cause serious health effect at high concentrations to humans [3-6]. Thus the need to removing them from water sources becomes imperative.Several methods have been developed for the removal of dyes and heavy metals from wastewaters. These include physiochemical, chemical and biological methods such as coagulation and flocculation [7], ozonation [8], electrochemical methods [9], fungal decolonization [10] and adsorption [11-14]. Amongst the techniques mentioned, adsorption is the method of choice because of its ease of operation and design. Recently, Layered double hydroxides (LDH), also known as hydrotalcites, have been widely used as adsorbent for various applications due to their unique anion exchange capability. In addition, the spent adsorbent can be easily regenerated and its adsorption capacities are comparable with the fresh LDH adsorbents [15-17]. In general, LDH consists of positively charged metal hydroxide sheets with anions located between the layers to compensate the positive layer charges. The composition of LDH is generally represented by the following equation [17-20]:
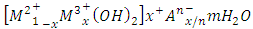 where M2+ represents divalent cations
where M2+ represents divalent cations 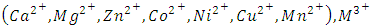 represents trivalent cations
represents trivalent cations 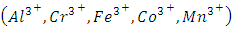 represents inorganic or organic anions
represents inorganic or organic anions 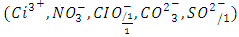 m is the number of interlayer water and
m is the number of interlayer water and 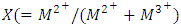 is the layer charge density of LDH. Due to their exchange capacity multiple uses have been adduced these hydrotalcites [21-23]. This work was aimed at successfully synthesizing Ni/Al-CO3, and hydrotalcite in the laboratory for the adsorption of dyes and heavy metals in wastewater, understanding that access to clean water is still a fundamental problem in the world.
is the layer charge density of LDH. Due to their exchange capacity multiple uses have been adduced these hydrotalcites [21-23]. This work was aimed at successfully synthesizing Ni/Al-CO3, and hydrotalcite in the laboratory for the adsorption of dyes and heavy metals in wastewater, understanding that access to clean water is still a fundamental problem in the world.2. Materials and Methods
- Carbonate form of Ni-Al LDH A 50ml aqueous solution containing 0.3M Ni(NO3)2.6H2O and 0.1M Al (NO3)3.6H2O with Ni/Al ratio 4:1, was added drop wise into a 50 ml mixed solution of (NaOH (2M) and Na2CO3 (1M) with vigorous stirring and maintaining a pH of greater than 10 at room temperature. After complete addition, the slurry formed was aged at 60°C for 18 and 24 hrs respectively. The products were centrifuged at 5000 rpm for 5 minutes, with distilled water three to four times and dried by freeze drying. The same procedure was followed to synthesize LDHs with Ni2+:Al3+ ratios of 3:1, 2:1 and 1:1 respectively. Characterization of the LDHsThe X-ray powder diffraction pattern of synthesized LDH was taken with a Scintag diffractometer operated at 35 kV voltage and 30 mA current using CuKα radiation. A step scan of 5° /min was used in the continuous method in a range of 5-40° 2θ. SEM measurements were performed using Hitachi S4100 with electron beam energy of 25 keV and Hitachi SU-70 scanning electron microscope (SEM) coupled with energy dispersive spectroscopy (EDS). LDHs for measurements were dispersed in ethanol or water using ultrasound and were later placed on the H-terminated silica on the holder. FT-IR absorption spectra of the samples were recorded in a Perkin-Elmer FT-IR spectrometer (Model-FT-1730). The powdered samples completely dried were ground with KBr in 1:20 sample ratio and pressed to form a disc. This disc was placed in the sample holder of the FT-IR for the IR determination. Sixty four (64) spectra (recorded with a nominal resolution of 4 cm-1) were accumulated and averaged to improve the signal-to- noise ratio.Preparation of Metal Solutions/Dyes SolutionsA stock solution of 1000mgl-1 was prepared by dissolving 1.127g Methylene blue in 1000ml distilled water. This gave the Methylene blue stock. The experimental solution was prepared by diluting the stock solution with distilled water to between 20 - 40mgl -1. The concentration of MB was determined at 630nm by the UV – visible spectrophotometer.The analytical grade Congo red dye supplied by Labchem Laboratory was used as received. Stock solution of the dye was prepared by dissolving 1g solute in 1000ml volumetric flasks to make 1000 mg/L of the dye solution. Model and working calibration standards were prepared by serial dilution of the stock solution to concentrations of 20, 30 and 40mgl-1.Stock solution of lead nitrate [Pb(NO3)2] was prepared by dissolving 3.312g in 100ml of de-ionized water to obtain concentrations of 0.13 g/L Pb, 0.25 g/L Pb and 0.38 g/L Pb for the adsorption studies.Working solutions of 0.08 g/L Cu, 0.12 g/L Cu and 0.16 g/L Cu were prepared by dissolving 1.6g of copper sulphate (CuSO4) in 100ml of de-ionized water for the various batch adsorption studies.Batch Adsorption Procedure A 0.2g each of the powdered sample was collected and weighed out using an electronic weighing balance; the weighed sample was placed in three (3) pre-cleaned tubes. 10ml of the dye solutions (MB and CR) with standard concentrations of 20mg/L made from the stock solution in 3.6 above was added to each test tube containing the weighed sample and equilibrated by shaking (agitation) for 1 hour at temperatures of 40C, 60C and 80C respectively using a compenstant Gallenhamp water bath. This was immediately centrifuged at 2500rpm for 5 minutes and then decanted. The supernatant were stored for dye UV-analysis. The same procedure was used for the analysis of metal ions (Pb2+ and Cu2+) from standard spectroscopic grade lead nitrate (Pb(NO3)2 and copper sulphate (CuSO4) using initial concentrations of 0.38g/L and 0.16g/L respectively. A 0.2g each of the powdered sample of LDH was weighed out using an electronic weighing balance and the weighed sample was placed in three (3) pre-cleaned test tubes. 10ml of each metal ion solution with standard concentrations for (0.13, 0.25, 0.38) g/L and (0.08, 0.12, 0.16) g/L for Cu2+ which were made from spectroscopic grade standards of lead nitrate (Pb(NO3)2) and copper sulphate (CuSO4) were added to each test tube containing the weighed sample and equilibrated by shaking (agitation) for 1 hour and then centrifuged at 2500rpm for 5 minutes and decanted. The supernatants were stored for the metal ion (Pb2+ and Cu2+) analysis. Concentrations of (20, 30 and 40) mg/L were used for methylene blue (MB) and congo red (CR) respectively. The supernatants were then stored for UV-analysis. A 0.2g each of the powdered sample of LDHs was weighed using an electronic weighing balance and placed in three (3) pre-cleaned test tubes. 10ml of the metal ion (Pb2+ and Cu2+) solutions with concentration of 0.35g/L and 0.16g/L made from spectroscopic grade standard of lead nitrate (Pb(NO3) and copper sulphate (CuSO4) were added to each test tube containing the weighed sample and equilibrated by shaking (agitation) for each time intervals of 10, 20 and 30 minutes respectively. The sample suspensions were centrifuged for 5 minutes at 2500rpm and decanted and the supernatants stored for metal ion (Pb2+ and Cu2+) analysis. For the time studies of dyes (MB and CR), initial concentration of 20mg/L were used for the time differential studies and supernatants store and used for UV-analysis.Metal AnalysisThe concentration of CR and MB in the experimental solutions were determined from the calibration curve prepared by measuring the absorbance of different known concentrations of CR and MB solutions at wavelengths of 497 nm and 680nm respectively using a UV-vis spectrophotometer (Shimadzu, Kyoto, Japan). The metal analysis (Pb2+ and Cu2+) was performed by AAS using a buck scientific atomic absorption Spectrophotometer 200A (AAS). Data Analysis The various experimental data were subjected to equilibrium, thermodynamics and kinetic analysis for proper interpretation of adsorption processes.Isotherms Equilibrium Analysis The interpretation of adsorption capacity (qe) and the sorption efficiency (R%) was done using equations 1 and 2 below:
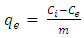 | (1) |
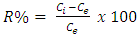 | (2) |
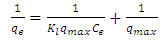 | (3) |
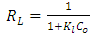 | (4) |
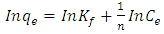 | (5) |
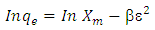 | (6) |
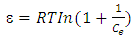 | (7) |
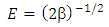 | (8) |
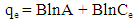 | (9) |
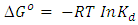 | (10) |
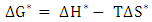 | (11) |
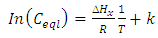 | (12) |
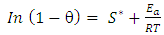 | (13) |
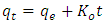 | (14) |
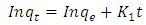 | (15) |
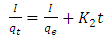 | (16) |
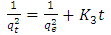 | (17) |
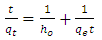 | (18) |
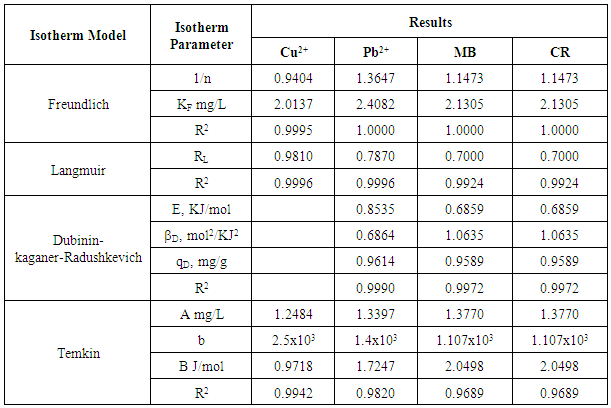
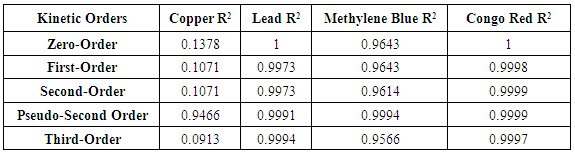
3. Results and Discussions
- Colours of Ni/Al-CO3 crystalThe colour of the crystals is the predominant greenish colour of nickel nitrate. However, the intensity decreases with decrease in Ni/Al ratio. Characteristics of Ni/Al-CO3Figures 1 and 2 show the pre-adsorption spectra of copper, lead, methylene blue and congo red and post-adsorption spectra of methylene blue and congo red on Ni/Al-LHD respectively. The strong band around 3400cm-1 as shown in figure 1 is associated with the stretching vibration of OH groups in the brucite like layer and interlayer molecules. The broadening of the band was attributed to the hydrogen-bond formation. Less intense absorption band around 1650 – 1500 cm-1 was assigned to the bending vibration of the interlayer water molecules. The carbonate ion peak is around 1400cm-1 which is consistent with layered double hydroxides. The sharp peaks of heterocyclic aromatic compounds are noticed between 900cm-1 - 700cm-1 in figure 2a. This is due to C-H out-of-plane vibration and is consistent with the vibration spectra of methylene blue. Figure 2b shows sharp peak around 733cm-1 which is characteristic of bending vibration of primary amine and phosphate bond stressing between 1100cm-1 - 1200cm-1. This shows adsorption actually occurs via formation of complex between the congo red and the layered double hydroxides [24, 25].
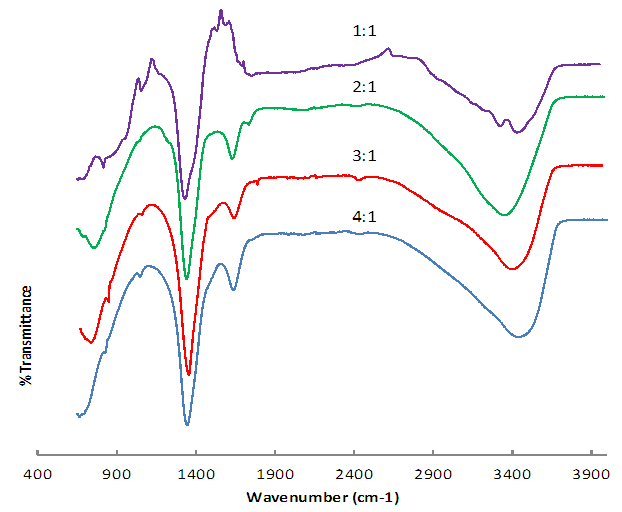 | Figure 1. Pre adsorption spectra of different ratios of Ni/Al-CO3 |
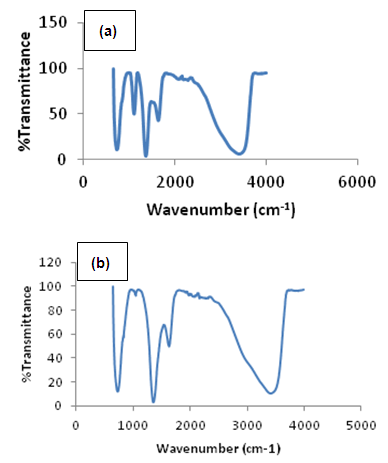 | Figure 2. Ni/Al-CO3 Fourier transform infrared spectroscopy: Post adsorption spectra of Methylene Blue (a) and Congo Reds (b) |
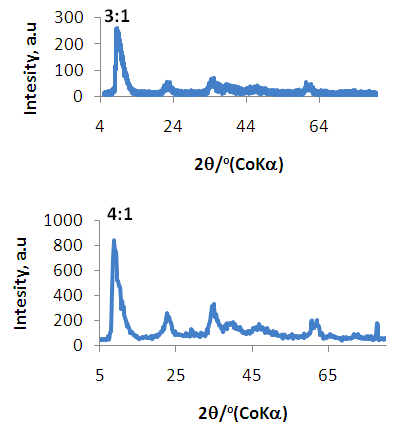 | Figure 3. XRD Spectra of Ni/Al-CO3 for Copper (3:1) and Lead (4:1) adsorption |
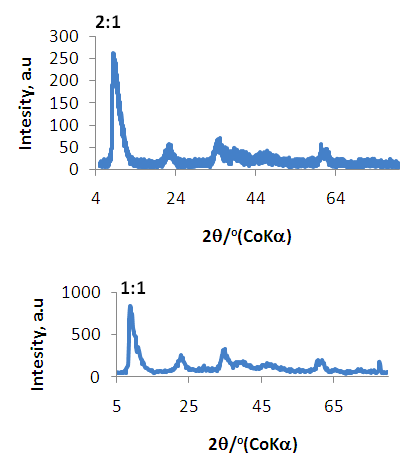 | Figure 4. XRD Spectra of Ni/Al-CO3 for Copper (2:1) and Lead (1:1) adsorption |
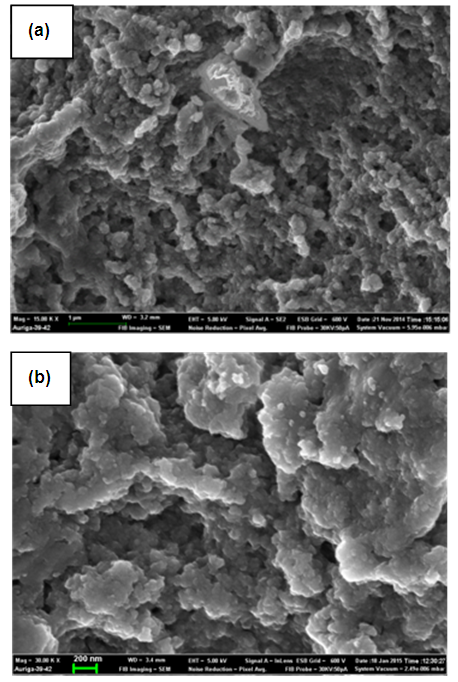 | Figure 5. Scanning Electron Microscope (SEM) micrograph of Ni/Al-CO3 before (a) and after (b) adsorption studies of Copper |
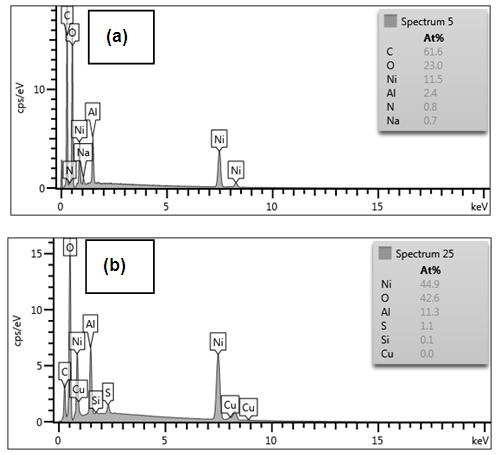 | Figure 6. Energy Dispersive Spectroscopy of Ni/Al-CO3: Pre and post Adsorption graphs indicating absence (a) and presence (b) of Copper |
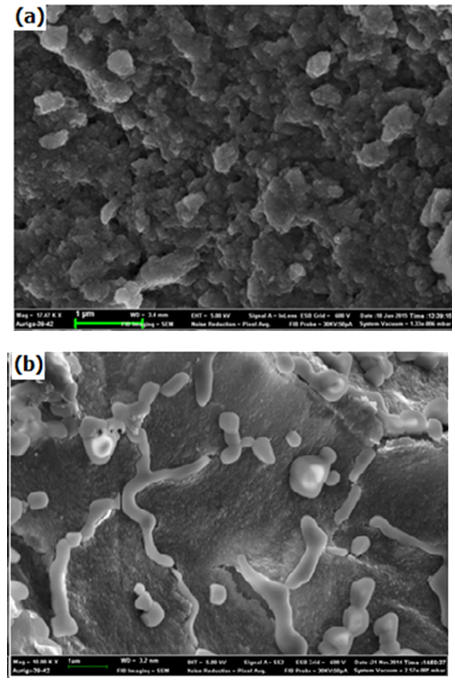 | Figure 7. Scanning Electron Microscope (SEM) micrograph of Ni/Al-CO3 before (a) and after (b) adsorption studies of Lead |
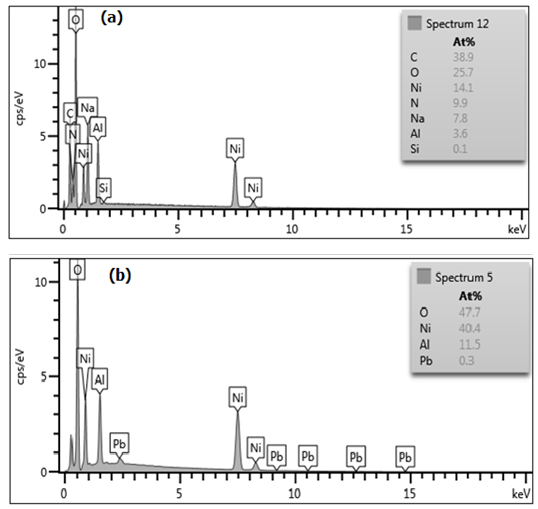 | Figure 8. Energy Dispersive Spectroscopy patterns of Ni/Al-CO3: Pre and post Adsorption graphs indicating absence (a) and presence (b) of Lead |
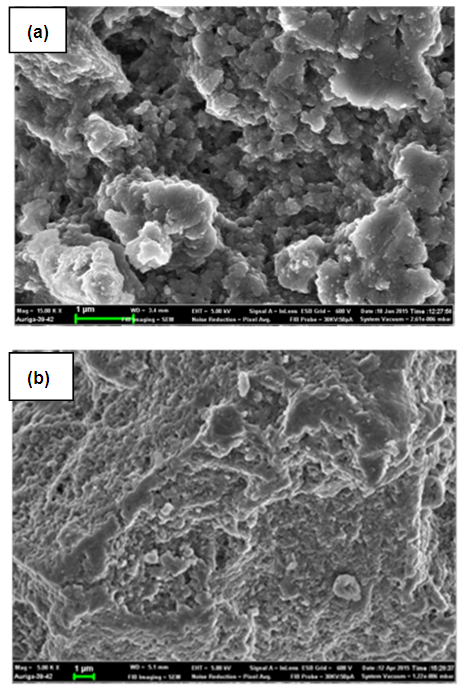 | Figure 9. Scanning Electron Microscope (SEM) micrograph of Ni/Al-CO3 before (a) and after (b) adsorption studies of Methylene Blue |
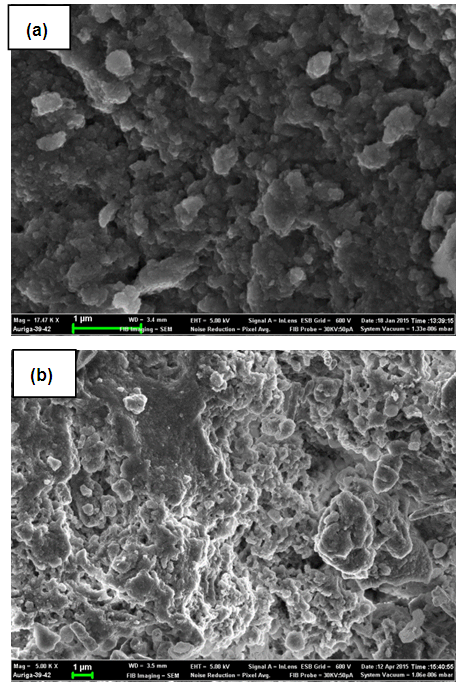 | Figure 10. Scanning Electron Microscope (SEM) micrograph of Ni/Al-CO3 before (a) and after (b) adsorption studies of Congo Red |
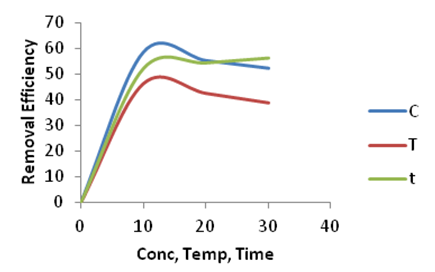 | Figure 11. Effect of Concentration (C), Temperature (T) and Time (t) on the adsorption of Copper onto Ni/Al-CO3 |
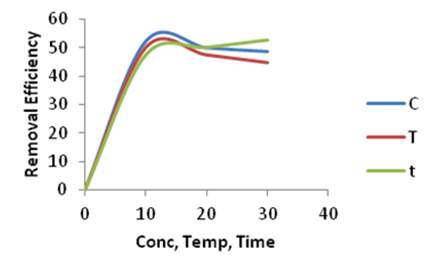 | Figure 12. Effect of Concentration (C), Temperature (T) and Time (t)on the adsorption of Lead onto Ni/Al-CO3 |
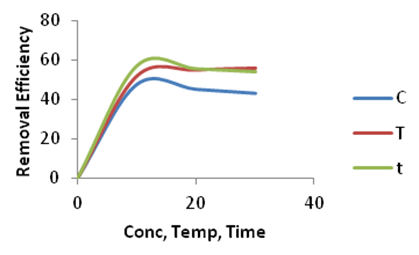 | Figure 13. Effect of Concentration (C), Temperature (T) and Time (t) on the adsorption of Methylene Blue onto Ni/Al-CO3 |
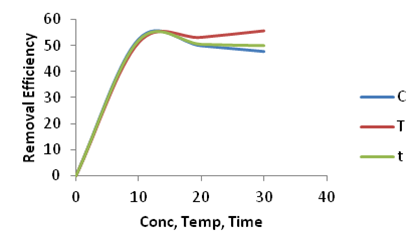 | Figure 14. Effect of Concentration (C), Temperature (T) and Time (t)on the adsorption of Congo Red onto Ni/Al-CO3 |
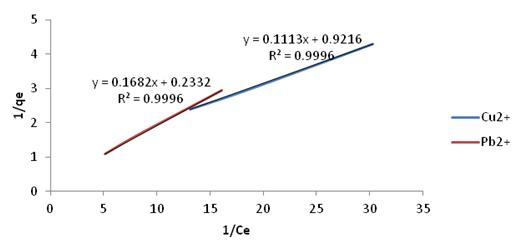 | Figure 15. Langmuir Isotherm plots for adsorption of Copper and Lead onto Ni/Al-CO3 |
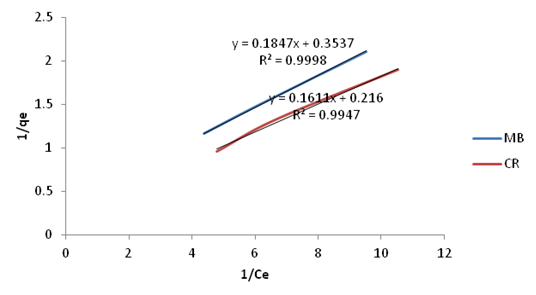 | Figure 16. Langmuir Isotherm plots for adsorption of Methylene Blue and Congo Red onto Ni/Al-CO3 |
|
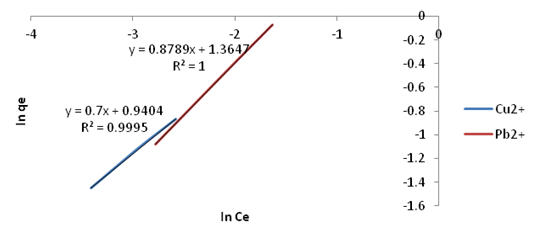 | Figure 17. Freundlich Isotherm plots for adsorption of Copper and Lead onto Ni/Al-CO3 |
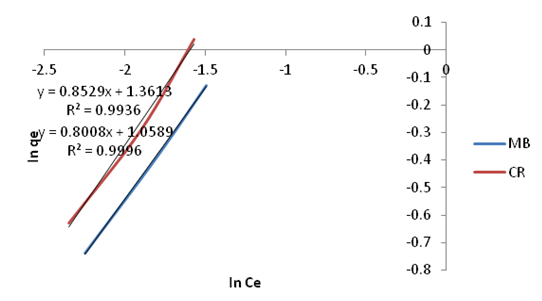 | Figure 18. Freundlich Isotherm plots for adsorption of Methylene Blue and Congo Red onto Ni/Al-CO3 |
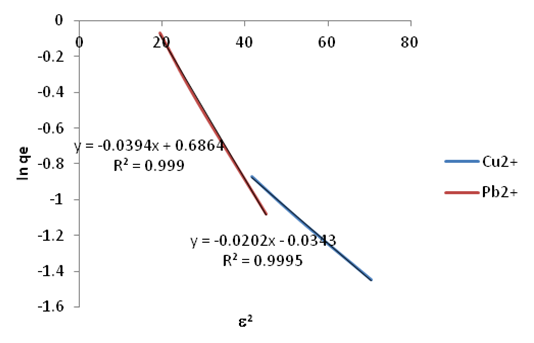 | Figure 19. Dubinin-Kaganer-Radushkevic (DKR) Isotherm plots for adsorption of Copper and Lead onto Ni/Al-CO3 |
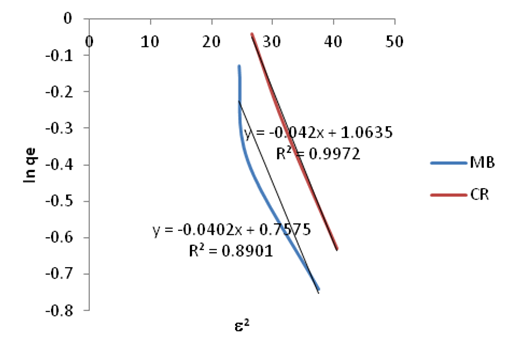 | Figure 20. Dubinin-Kaganer-Radushkevic (DKR) Isotherm plots for adsorption of Methylene Blue Congo Red onto Ni/Al-CO3 |
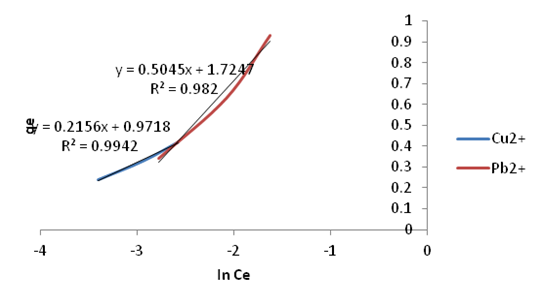 | Figure 21. Temkin Isotherm plots for adsorption of Copper and Lead onto Ni/Al-CO3 |
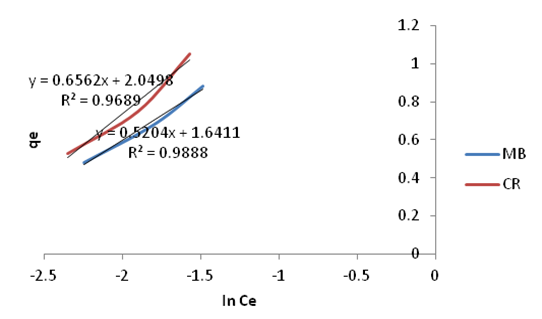 | Figure 22. Temkin Isotherm plots for adsorption of Methylene Blue and Congo Red onto Ni/Al-CO3 |
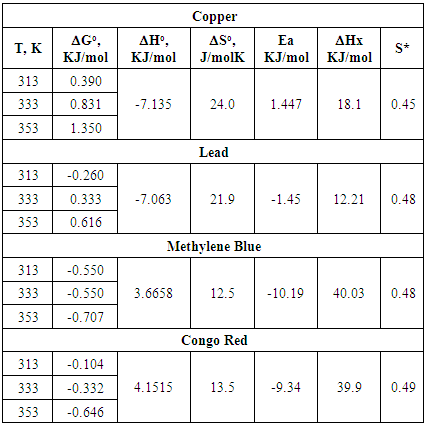
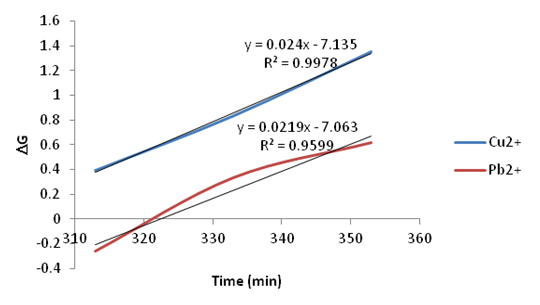 | Figure 23. Plots of Go vs. Temperature for the adsorption of Copper and Lead onto Ni/Al-CO3 |
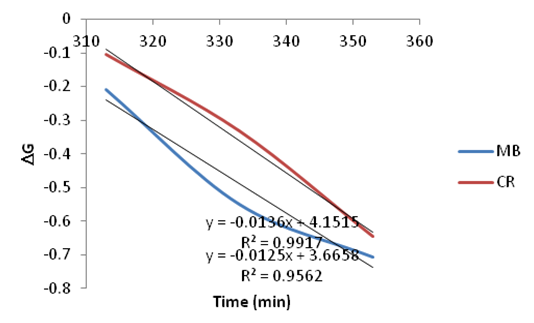 | Figure 24. Plots of Go vs. Temperature for the adsorption of Methylene Blue and Congo Red onto Ni/Al-CO3 |
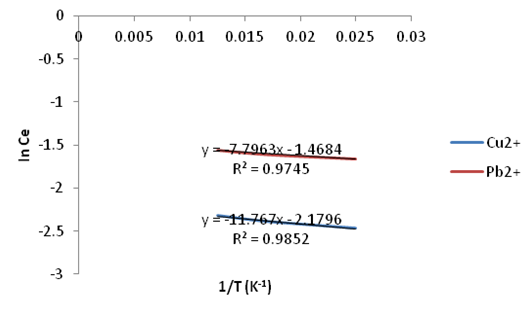 | Figure 25. Plots of In Ce vs. 1/T for the adsorption of Copper and Lead Red onto Ni/Al-CO3 |
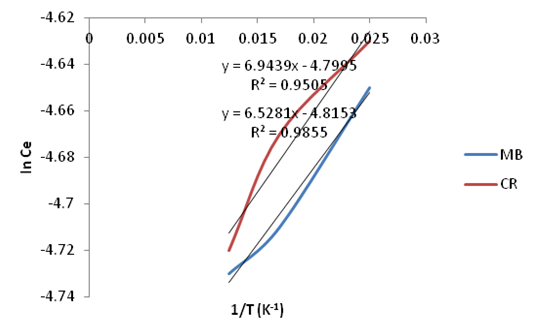 | Figure 26. Plots of In Ce vs. 1/T for the adsorption of Methylene Blue & Congo Red onto Ni/Al-CO3 |
|
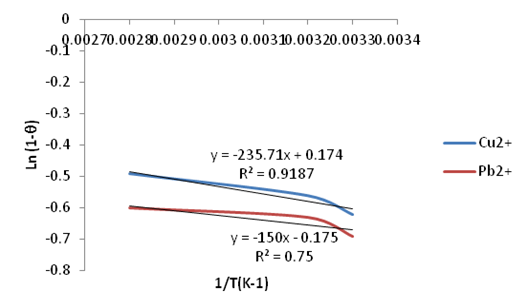 | Figure 27. Plots of Ln(1-θ) vs. 1/T(K-1) for the adsorption of Copper and Lead Ni/Al-CO3 |
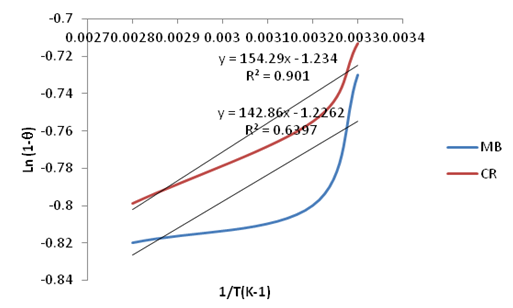 | Figure 28. Plots of Ln(1-θ) vs. 1/T(K-1) for the adsorption of Methylene Blue and Congo Red onto Ni/Al-CO3 |
|
4. Conclusions
- It can be concluded that Ni/Al-CO3 can be employed as adsorbent for the removal of Lead (II), copper (II), methylene blue (MB) and congo red (CR) from aqueous solutions. However, contact time, temperature and initial concentration of dyes and metals could either affect the adsorption capacities of the layered double hydroxide positively or negatively depending on the range of the parameters. The application of the experimental data in defining equilibrium and thermodynamic parameters is consistent with physical adsorption. This indicates that Ni/Al-layered double hydroxide is indeed a novel adsorbent.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML