-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2017; 7(1): 5-13
doi:10.5923/j.ijmc.20170701.02

The Use of Rubber leaf Extract as a Corrosion Inhibitor for Mild Steel in Acidic Solution
1Chemical Engineering Department, Federal University of Petroleum Resources, Effurun, Nigeria
2Chemical Engineering Department, Nnamdi Azikiwe University, Awka, Nigeria
Correspondence to: Okewale A. O., Chemical Engineering Department, Federal University of Petroleum Resources, Effurun, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This work has explored the possibility of using plant extract as a corrosion inhibitor other than the use of conventional organic materials as corrosion inhibitor. Rubber leaf extract which contains the carbonyl groups, aromatic rings, and double bonds as indicated by the GCMS, phytochemical, and FTIR analysis is one of the good natural plant extract as corrosion inhibitor. Gravimetric method was used to determine the weight loss on surface of mild steel at various inhibitor concentrations in 0.1M HCl solution. The highest inhibition efficiency of 86% was achieved using the rubber leaf extract as inhibitor. Adsorption mechanism was investigated using Langmuir, Temkin, and Freundlich isotherms. Inhibitor adherence on the mild steel surface was spontaneous with the negative Gibb’s free energy value of -18.07kJ/mol obtained. The inhibitor was adsorbed on the mild steel surface through adsorption of the phytochemical components on the surface of mild steel which protects the metal surface from corroding. The corrosion rate decreases with increase in various inhibitor concentrations and exposure time studied.
Keywords: HCl, Weight loss, Corrosion, GCMS, FTIR, Phytochemical, Inhibitor
Cite this paper: Okewale A. O., Olaitan A., The Use of Rubber leaf Extract as a Corrosion Inhibitor for Mild Steel in Acidic Solution, International Journal of Materials and Chemistry, Vol. 7 No. 1, 2017, pp. 5-13. doi: 10.5923/j.ijmc.20170701.02.
Article Outline
1. Introduction
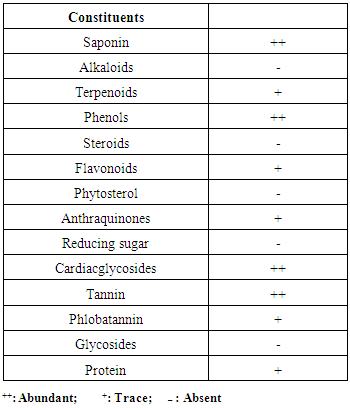
- Corrosion of metals and alloys particularly in acidic media is an important industrial problem (Ghulamulla et al., 2015). Mild steel is one of the industrially important metals used under different conditions in Chemical and allied, and Oil and Gas industry in handling acid, and alkaline conditions. Use of corrosive chemicals in industries is unavoidable, which could lead to the dissolution of the metal (Noyel et al., 2015). The use of some non – conventional materials as corrosion inhibitors to replace the hazardous synthetic corrosion inhibitors prompted research in ecofriendly inhibitors. The non-toxic, biodegradable, availability, anti – oxidants and cheapness of these natural organic materials make them suitable for use as environmentally corrosion inhibitors (Nwigbo et al., 2012). Corrosion is a naturally occurring phenomenon which deteriorates a metallic material or its properties because of a reaction with its environment, it can cause dangerous and expensive damage to everything from pipelines, bridges and public buildings to vehicles, water and wastewater systems, and even home appliances and thus is one of the most serious problems in the industry especially the oil and gas industry (Anbarasi et al., 2011). Corrosion is something we hope to avoid; but ultimately it is something we must learn to deal with (Shaw and Kelly, 2006). The prevention of corrosion in steel has played a pertinent role in various industries, especially in the chemical and petrochemical processing industries that employ the use of steel thus, a number of studies have been and is being conducted to investigate effective methods for preventing corrosion (Al – Ameiry et al., 2014). Approaches available for controlling corrosion include the application of protective coatings to metal surfaces, the addition of chemical species to the environment to inhibit corrosion, alteration of alloy chemistry, and the treatment of the surface of a metal. Although, recent trends in corrosion research now include the development of environmentally friendly inhibitors, accurate prediction of structure service life, and finding ways to make corrosion a good thing (Shaw and Kelly, 2006). The corrosion inhibitor is one of the best known methods of corrosion protection and one of the most useful in the industry due to its low cost and practice method (Dariva and Galio, 2014). The chemicals that can act as corrosion inhibitors may be inorganic or organic (Al-Amiery et al., 2014). Adsorption of molecules and ions on the metal surface is effectively done by organic inhibitors compound. The presence of large molecules with functional groups containing of hetero – atoms (such as oxygen, nitrogen, sulphur, and phosphorus), triple bonds or aromatic rings in the inhibitor’s chemical structure enhance the adsorption process (El – Etre, 2007). The formation of passivating layer that prevents access of corrosive agent onto the steel surface, either by inhibiting the reduction or oxidation part of the redox reaction or by dissolution of oxygen and scavaging is one of the corrosion inhibitive mechanisms of a good corrosion inhibitor. Plants represent a class of interesting source of compounds currently being explored for use in metal corrosion protection in most systems, as possible replacement of toxic synthetic inhibitors. Hence, rubber leaf extract has a good characteristic as a corrosion inhibitor owning to the fact that it contains inhibitive components such as phenols, tannins, saponins, anthraquinones, cardiacglycosides, and phlobatannin. There are virtually little or no reports in literature on the use of rubber leaf extract as a corrosion inhibitor on mild steel which necessitated this study.
2. Materials and Experimental Procedure
- The material studied was mild steel. The sample, rubber leaf was collected from rubber tree plantation located at Ugbomro, Community in Delta State, Nigeria. The sample leaf was washed, shred and sun dried. The HCl, acetic acid, and ethanol solutions were analytical grades obtained from an accredited chemical dealer in Warri, Delta State, Nigeria. The leaf sample was sun dried and grinded into powdery form and thereafter classified with 600µm mesh sieve.Extraction of rubber leaf extractA 500ml soxhlet apparatus and ethanol as solvent was used for the extraction of rubber leaf extract. Some quantity of the powdered sample is poured on a Mushin cloth and placed inside the thimble of the apparatus. A round bottom flask containing known volume of ethanol is fixed to the end of the apparatus and the condenser is tightly fixed at the bottom end of the extractor. The whole set up is heated on a heating mantle at a temperature of 70°C. The solvent was allowed to remain in contact with the powdery rubber leaf material for 12 hr and the excess solvent in the oil was recovered by heating. Experimental procedureThe weight loss test method was used to study the corrosion inhibitor. The mild steel (specimens) were manually polished with silicon carbide abrasive paper, greased to avoid further corrosions and degreased with petroleum ether, washed in distilled water and dried. Each metal coupon is size 2cm x 3cm x 0.12cm. Before polishing, a hole of about 0.4cm was drilled on each coupon. The coupon is suspended with the aid of nylon thread and glass rod in a 100ml beaker with 100ml of the 0.1M HCl without and with different concentrations of the inhibitor. To prevent evaporation of solution and contamination, the corrosion vessel was covered with paraffin. At various time intervals of 5, 24, 48, 72, 96 and 120 hours, and inhibitor concentrations of 0.1g, 0.5g, 1.0g, and4.0g/ 100ml of 0.1M HCl solution at room temperature (30°C), dipped in distilled water and immersed in methanol solution which was scrubbed with bristle brush, to remove residual acids, and inhibitors concentration and then washed with washing liquor (NaOH + Zn dust) thoroughly, rinsed with distilled water, and later dried in acetone before reweighed (Nwigbo et al., 2012).Effect of inhibitor concentration on corrosionThe effect of inhibitor concentration on corrosion was investigated by changing the initial inhibitor concentration in the range 0.1g, 0.5g, 1.0g, and4.0g/ 100ml of 0.1M HCl, at room temperature for different hours.Effect of time of exposureThe effect of exposure time on corrosion was investigated for 5, 24, 48, 72, 96 and 120 hours /100ml of inhibitor to 0.1M HCl concentration.Determination of the functional group present in rubber leaf extractGC-MS (Gas Chromatography Mass Spectroscopy) Analysis.Gas chromatography mass spectroscopy (GC MS – QP2010) plus SHIMADZU was carried out, to detect all the organic components present in the rubber leaf extract. FTIR (Fourier Transform Infrared Spectroscopy) Analysis.The structural organization of the rubber leaf extract was investigated to identify the functional group present. The rubber leaf extract was examined using (FTIR – 8400S) SHIMADZU spectrophotometer model with the range 500 – 4000cm-1 (wavelength). KBr (potassium bromate) is used as a background material in the analysis. Determination of the phytochemical constituents in rubber leaf extract The phytochemical screening of the rubber leaf extract was done to identify the main groups of active chemical constituents present in the leaf extract by their colour reaction. The presence of saponins, alkaloids, terpenes, flavonoids, glycosides, reducing sugars and tannins were tested for by the simple qualitative and quantitative methods of (Herborne, 1973; Okwu, 2001; Rahilla et al., 1994; Sofowora, 1993; and Odeja et al., 2015). Test for saponins 0.2g of extracts was shaken with 5ml of distilled water in a test tube. Frothing which persists on warming was taken as evidence for the presence of saponins.Test for alkaloids0.2g of extracts was shaken with 1% HCl for two minutes. The mixture was filtered and drops of Wagner’s reagent added. Formation of a precipitate indicated the presence of alkaloids. Test for phenols0.2g of extracts was dissolved in ferric chloride solution. A green or dirty green precipitate indicated the presence of phenolic compound. Test for steroids (Salkowski’s test)0.2g of the extracts was dissolved in 2ml of chloroform. Concentrated sulphuric acid was carefully added to form a lower layer. A reddish-brown colour at the interphase indicated the deoxy sugar characteristics of cardenolides. Test for flavonoids0.5g of magnesium powder and two drops of concentrated hydrochloric acid were added to 3ml of the extracts. A red or intense red colouration indicated the presence of flavonones. Test for anthraquinones0.2g of the extracts was shaken with 4ml of benzene. The mixture was filtered and 2ml of 10% ammonia solution was added to the filtrate. The mixture was shaken and the presence of pink, red or violet colour in the ammoniacal (Lower) phase indicated the presence of free anthraquinones. Test for reducing sugars0.2 g of the extracts was shaken with distilled water and filtered. The filtrate was boiled with drops of Fehling’s solution A and B for two minutes. An orange precipitate on boiling with the Fehling’s solution indicated the presence of reducing sugars. Test for cardiac-active glycoside (Keller-Killani Test)0.2 g of the extracts was dissolved in 2ml of glacial acetic acid containing one drop of ferric chloride solution followed by the addition of 1ml of concentrated sulphuric acid. A brown ring at the interface confirmed the presence of cardiac glycoside. Test for tannins0.2g of extracts was stirred with distilled water and filtered. Ferric chloride was added to the filtrate. A blue- black, green or blue-green precipitate was taken as an evidence for the presence of tannins. Test for phlobatanninsThe extracts (0.5g) was dissolved in distilled water and filtered. The filtrate was boiled with 2% HCl solution. Red precipitate shows the presence of phlobatannins. Test for glycosidesThe extracts was hydrolyzed with HCl solution and neutralized with NaOH solution. A few drops of Fehling’s solution A and B were added. Red precipitate indicates the presence of glycosides. Determination of inhibitor efficiencyThe inhibition efficiency was determined using the relationship below;
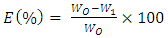 | (1) |
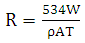 | (2) |
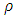 is the mild steel density, and A is the exposed area of corrosion.Determination of surface coverageThe degree of surface coverage (θ) is calculated from the weight loss measurement results using the following formula;
is the mild steel density, and A is the exposed area of corrosion.Determination of surface coverageThe degree of surface coverage (θ) is calculated from the weight loss measurement results using the following formula;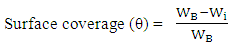 | (3) |
3. Results and Discussion
- The presence of the tannins, phenols, saponin, and anthraquinones with functional group containing (nitrogen, Oxygen and carbon), aromatic rings present in phenols constituent in the rubber leave extract chemical structure as depicted by the phytochemical test in Table 1 also enhance the process of corrosion inhibitor adsorption on the mild steel. This also corroborates the work of (Nwigbo et al., 2012; Prithiba et al., 2014; Owate et al., 2014). The presence of these compounds has been reported to promote the corrosion inhibition of mild steel in aggressive acid media (Umoren et al., 2006). Molecules containing nitrogen and acetylenic alcohols are claimed to form a film on the metal surface and can retard the metal dissolution process (an anodic reaction) as well as hydrogen evolution (a cathodic reaction) (Barmatov et al., 2012). Figure 1 gives the FTIR result of the rubber leaf extract. It can be seen that frequency (3362cm-1) depict RO-H(Alcohol) wide rounded broad band, OO-H (Carboxylic acid) group very wide band was shown with frequency (2919cm-1) while (2851cm-1) frequency correspond to C-H(p3) stretch of N-CH2. The broad band (1636cm-1) corresponds to C = O stretch ketone, R2C = O. The broad band 1513cm-1 revealed the primary amines, NH bond. The broad band of (1457cm-1) showed the presence of C-H bend alkyl group (Okewale et al., 2016). The presence of the carbonyl double bond carbon group in the rubber leave extract also suggests it to be a good corrosion inhibitor on mild steel in an acidic medium.
|
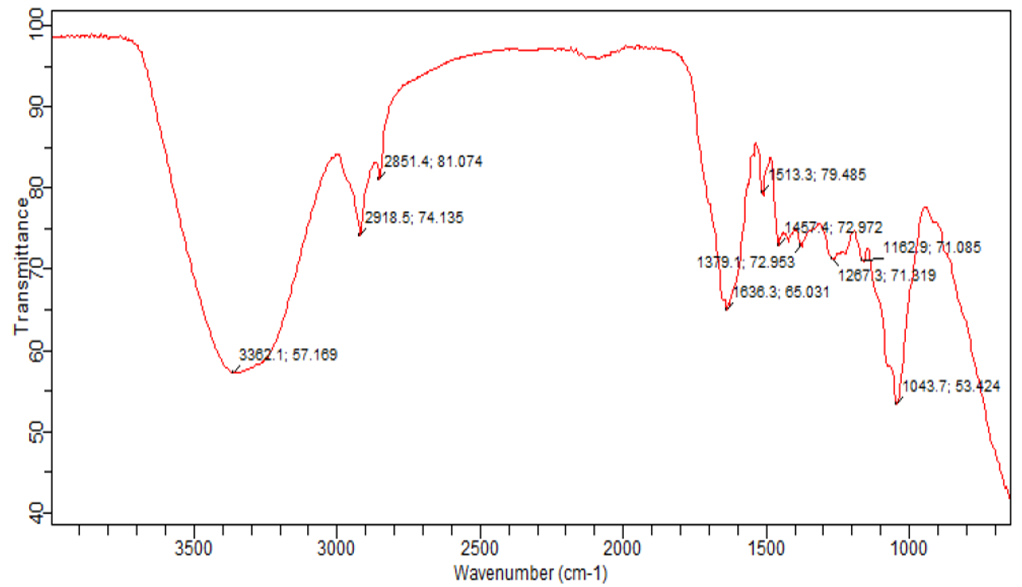 | Figure 1. FTIR Result of the Rubber Leaf Extract |
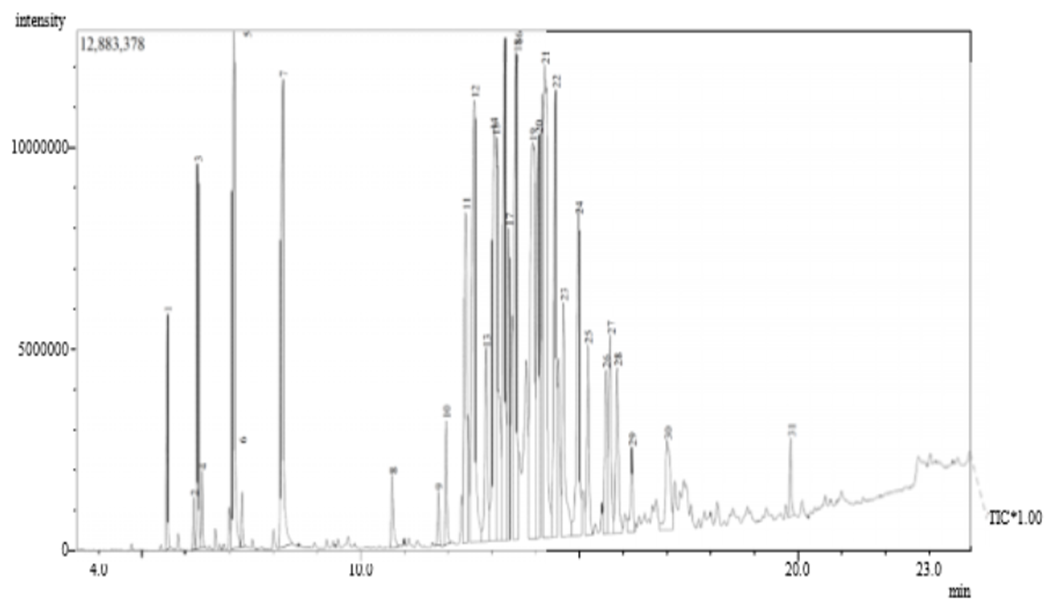 | Figure 2. GCMS of the Rubber leaf Extract |
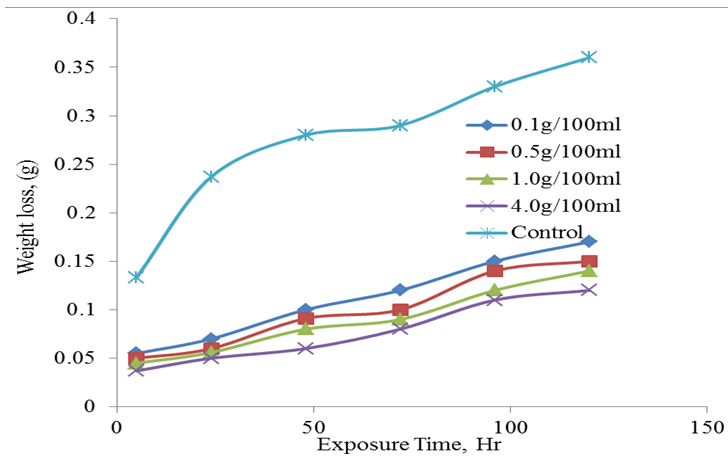 | Figure 3. Weight loss (g) against time of exposure (Hr) |
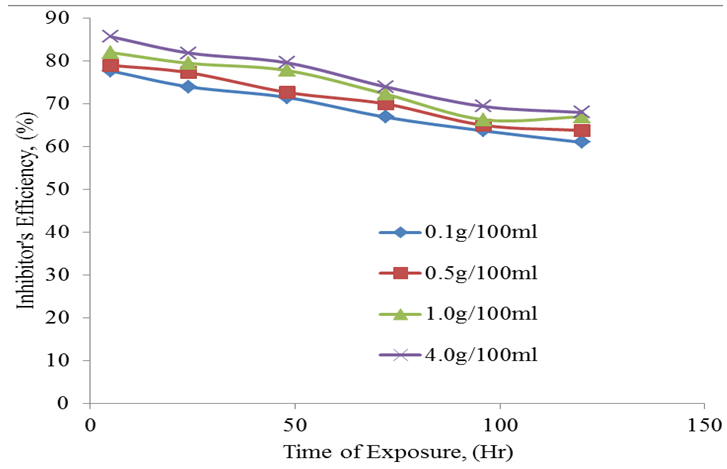 | Figure 4. Inhibitor’s efficiency against time of exposure |
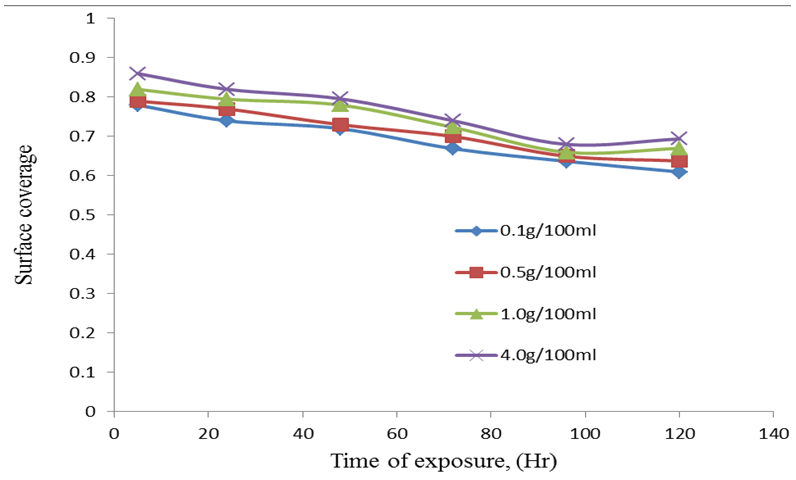 | Figure 5. Surface coverage against time of exposure |
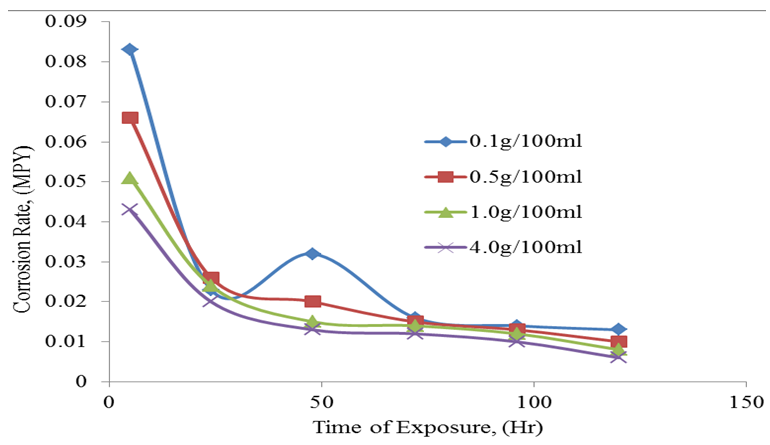 | Figure 6. Corrosion rate against time of exposure |
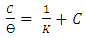 | (4) |
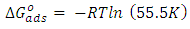 | (5) |
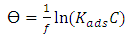 | (6) |
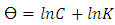 | (7) |
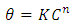 | (8) |
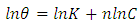 | (9) |
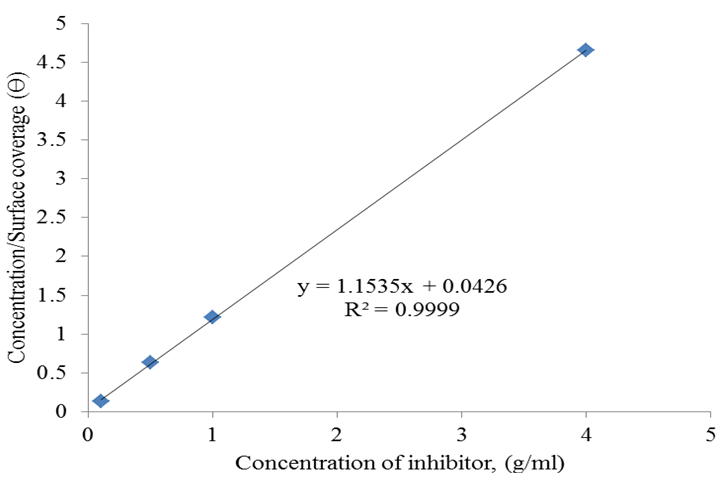 | Figure 7. Langmuir Adsorption Isotherm Plot for Rubber Leaf Extract Inhibitor on Mild Steel |
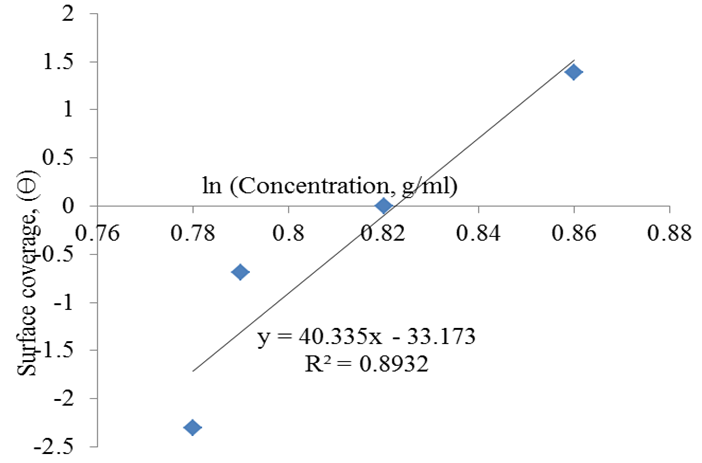 | Figure 8. Temkin isotherm plot of rubber leaf extract inhibitor on mild steel |
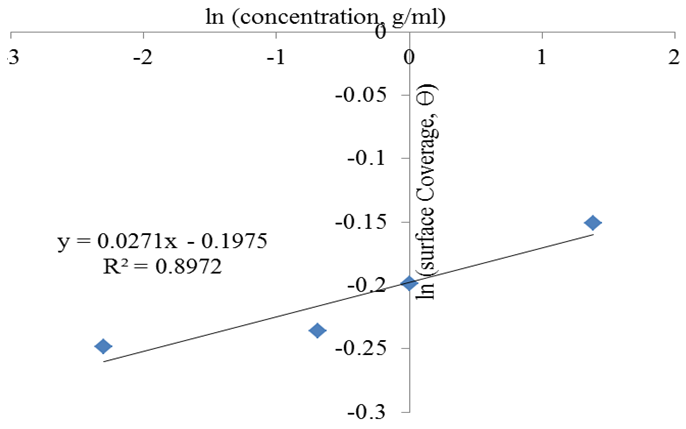 | Figure 9. Freundlich Isotherm for rubber leaf extract inhibitor on mild steel |
4. Conclusions
- The weight loss method was used to study the inhibitive potential of rubber leaf extract in acidic medium. The presence of tannins, saponins, anthraquinone, and flavonoids in the phytochemical analysis of the rubber leaf extract suggest it to be a good corrosion inhibitor on mild steel. The GCMS analysis and the FTIR result with double bond carbon functional group confirmed that the rubber leaf extract is a good corrosion inhibitor on mild steel surface in 0.1M HCl studied.86% inhibitor efficiency was achieved using the rubber leaf extract as corrosion inhibitor on mild steel. The inhibition efficiency of the extract decreases with time of exposure. The corrosion rate decreases with increase in inhibitor concentrations for the rubber leaf extract on mild steel surface. The corrosion rate decrease from 0.083 to 0.006MPY for the mild steel in the presence of rubber leaf extract inhibitor. The rubber leaf extract film was formed on the surface of mild steel by obeying Langmuir adsorption isotherm compared to Temkin, and Freundlich isotherms. The adsorption process is spontaneous and form monolayer on the surface of mild steel.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML