-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2017; 7(1): 1-4
doi:10.5923/j.ijmc.20170701.01

The Study of the Kinetics of Gel-Formation of the Polycaproamide-Silica Nanocomposite Material Obtained by the Sol-Gel Method
Furkat U. Yunusov1, Bakhodir D. Kabulov2, Khamdam I. Akbarov1, Ravshankhon Yu. Makhmudov2
1Department of Chemistry, The National University of Uzbekistan, Tashkent, Uzbekistan
2State unitary enterprise “Science end progress”, Tashkent State Technical University, Tashkent, Uzbekistan
Correspondence to: Furkat U. Yunusov, Department of Chemistry, The National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article provides the studies of the kinetics of gel-formation of the polycaproamide-silica nanocomposite materials, obtained by the sol-gel method based on tetraethoxysilane, polycaproamide, hydrochloride-L-lysine and the alcohols. It is shown that a significant effect on the process of gel-formation, investigated by a fluidity loss of reaction solutions, is depended on both molar ratios of initial components of reaction system and the quantity of added alcohol (ethanol or glycerol) introduced separately or as their mixture in the different ratios.
Keywords: Polycaproamide, Tetraethoxysilane, Silica, Sol-Gel, Nanocomposite material, Gel-Formation
Cite this paper: Furkat U. Yunusov, Bakhodir D. Kabulov, Khamdam I. Akbarov, Ravshankhon Yu. Makhmudov, The Study of the Kinetics of Gel-Formation of the Polycaproamide-Silica Nanocomposite Material Obtained by the Sol-Gel Method, International Journal of Materials and Chemistry, Vol. 7 No. 1, 2017, pp. 1-4. doi: 10.5923/j.ijmc.20170701.01.
Article Outline
1. Introduction
- In all over the world the interest to investigations and elaborations in the field of nanotechnologies as well as to the questions, related to them, of obtaining and investigation of nanomaterials and first of all nanocomposites has been increased in the last 10-15 years. The row of the new nanomaterials, such as fullerenes, nanotubes, quantum points and nanocomposite materials, was appeared. For the understanding the peculiarities of nanostate, the methods of obtaining and investigation of nanocompositional systems at decision of practical tasks investigators have devoted their attention to investigation of regularities and mechanisms of nanostructures formation in different inorganic and organic composite’s systems, their stabilization; determination of correlation between synthesis, structure and properties and also some technological process and ways of application of such materials [1, 2].Hybrid polymer-silica nanocompositional materials are a creative alternation for obtain materials possessed by specifically properties owing to which they are interested for such ranges of application as optical, electronics, mechanics, catalysis, obtain of membranes, protective covers, catalysts, sensors and also sorption materials of different purpose [3, 4].Using of sol-gel process is an ideal approach to obtain hybrid polymer-silica nanocompositional materials with different hierarchical porous structure owing to possibility of including in reaction solution of different organically compounds which are used as templates. The main dignities of such method are simplicity of equipment and mild temperatures conditions (as a rule room temperature) of it carrying out. Also an important role is the possibility of obtain of hybrid material in any desirable form: monoliths, films, powders [5, 6].Currently the water (hydrolytic) and the waterless (non-hydrolytic) sol-gel processes are used for the production of silica from the alkoxysilanes (tetraethoxy- or - tetramethoxysilanes). The hydrolytic path, become traditional, is based on the hydrolysis reactions catalysed by acid or base and the condensation of the products of hydrolysis occurring in organic solvents in the presence of water. In the waterless sol-gel process the formation of Si-O-Si siloxane bonds is due to the condensation reactions between substituted and unsubstituted alkoxysilanes with the separation of low molecular weight compounds [7-9].Understanding the base chemical reactions carrying out in sol-gel process has an important role for rational design and obtain of nanocompositional materials because this has allowed to regulate process beginning from choice of initial materials (precursors), added components, solvents and catalysts before obtained products.
2. Experimental
2.1. The Objects and the Methods of the Research
- We carried out a one-pot sol-gel process in a reaction sys-tem, consisting of tetraethoxysilane (TEOS), polycaproamide (PCA), Hydrochloride-L-lysine (HClL) and alcohols (ethanol and glycerol) dissolved in formic acid, and obtained polycaproamide-silica nanocomposite material.For that the 5% polycaproamide solution in formic acid was prepared. Further, the calculated amount of HClL, ethanol and glycerol, and then tetraethoxysilane were introduced into polycaproamide solution. The solution was subjected to ultrasonic treatment in an ultrasonic bath for 3 minutes for a better homogenization.Depending on the ratio of the components in the sol-gel reaction, after a time the formation of clear gel was begun.
3. Results and Discussion
- Obtained experimental results have shown that at one-reactor “waterless” sol-gel process in reaction systems consisting from TEOS, PCA, HClL, formic acid used as solvent and catalyst and also ethanol or glycerine included in process as additives complex interaction both physical and chemical nature have carried out including colloid-chemical interactions which were carried out to forming of hybrid polymer-silica nanocompositional materials. Knowing of bases of sol-gel process in this complex reaction system it is very important for regulation of properties of obtained materials.It is known that kinetics of gel-formation at sol-gel process of TEOS is depended on of rate of TEOS hydrolysis and condensation of intermediate products oxisilans [10-12]. When reaction of TEOS hydrolysis has prevailed than sol-gel process has carried out slowly and contrary when reaction of polycondensation has prevailed the sol-gel process has carried out with high rate.Also it is necessary to note that kinetics of reactions of hydrolysis and polycondensation in great degree has depended on ratio of initial reagents because in every case concrete stereochemical situation is formed: from one hand hydrolysis of TEOS and following polycondensation of byproducts - oxysilanes and on the other acts of formation of centres of origin of primary particles of silica. Grown of base structure-forming elements in reaction solution has carried out owing to reaction of polycondensation of surface OH - silanolic groups of colliding particles activity of which is depended on influence of presented in solution macromolecules what is shown from time of gel-formation in reaction system consisting from reagents TEOS and PCA taking in different molar ratios. From dependence on time of gel-formation from molar ratio TEOS:PCA presented in figure 1 it is shown that the more content of PCA the slower process of gel-formation was carried out.Slowing of gel-formation with increasing of PCA content (Figure 1) can be explain by screening influence on the silica particles of PCA macromolecules which have presented to approach of particles of silica sol at forming of silica net. However, time of gel-formation of such solution can be decreased by addition HClL, sample can be used as example in presence of HClL the time of gel-formation is equalled 27 min, but for sample didn’t containing HClL the time of gel-formation is equalled 91 min.
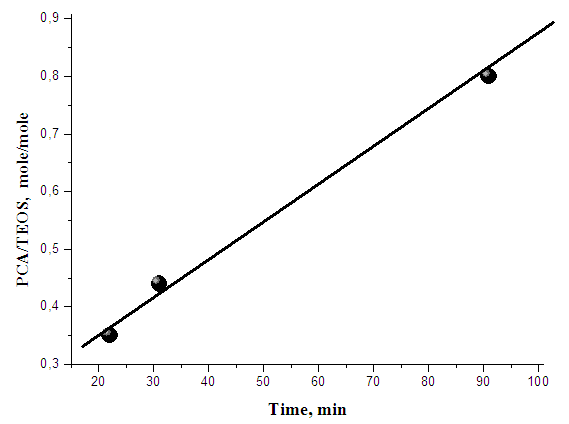 | Figure 1. Dependence on time of fluidity loss of solution of reagent TEOS in formic acid from content of PCA |
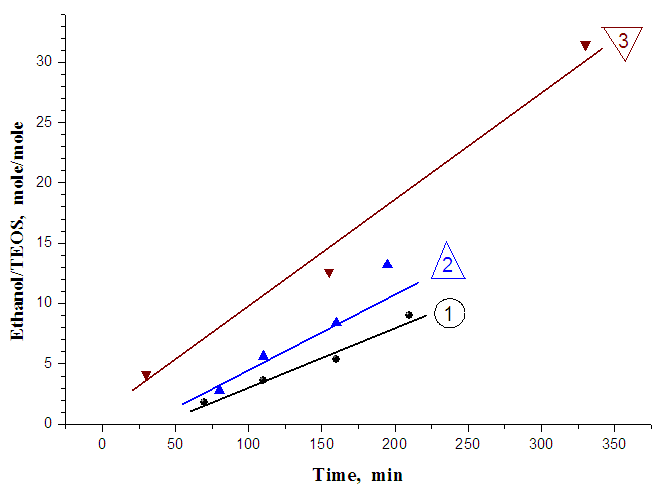
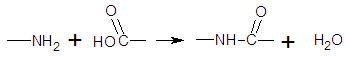 Presence in reaction solution of water molecules and catalytically active amino-groups on the ends of macromolecules of copolymer PCA and HClL (PCL) has allowed to explain mechanism of rapid formation of silica gel.At addition of ethanol in solution of PCA and HClL in formic acid in absence of TEOS the formation of gel was observed during 30 min what was witnessed about forming new polymer colloidal system in result of formation of sol particles from PCL. Formed such colloidal solution in following experiments is a new dispersion medium for sol-gel process of TEOS. Results of investigations have shown that addition of ethanol, glycerin or their mixtures in reaction medium has influenced on the time of gel-formation. For example, at the same mole ratio of initial reagents TEOS, PCA and HClL the time of gel-formation for reactional mixture with ethanol was equaled 155 min; with glycerin – 3 min and at addition of their mixture the time of gel-formation was in 2-time rapidly than in the presence of ethanol and was much slower than in the presence of glycerin. Consequently, by regulation of quantity of added alcohols in reaction mixture it is possible to optimize the duration of process of gel-formation. Investigations by study of sol-gel process at different compositions of reaction systems consisting from initial TEOS, PCA and HClL and also containing of different quantities of ethanol, glycerin and their mixtures have been carried out more detail.Results of investigation of influence of ethanol content in reaction solution at different ratios of initial reagents to one mole of TEOS have shown that with increasing of ethanol content the time of fluidity loss has increased and consequently the time of gel-formation also has increased (Figure 2).
Presence in reaction solution of water molecules and catalytically active amino-groups on the ends of macromolecules of copolymer PCA and HClL (PCL) has allowed to explain mechanism of rapid formation of silica gel.At addition of ethanol in solution of PCA and HClL in formic acid in absence of TEOS the formation of gel was observed during 30 min what was witnessed about forming new polymer colloidal system in result of formation of sol particles from PCL. Formed such colloidal solution in following experiments is a new dispersion medium for sol-gel process of TEOS. Results of investigations have shown that addition of ethanol, glycerin or their mixtures in reaction medium has influenced on the time of gel-formation. For example, at the same mole ratio of initial reagents TEOS, PCA and HClL the time of gel-formation for reactional mixture with ethanol was equaled 155 min; with glycerin – 3 min and at addition of their mixture the time of gel-formation was in 2-time rapidly than in the presence of ethanol and was much slower than in the presence of glycerin. Consequently, by regulation of quantity of added alcohols in reaction mixture it is possible to optimize the duration of process of gel-formation. Investigations by study of sol-gel process at different compositions of reaction systems consisting from initial TEOS, PCA and HClL and also containing of different quantities of ethanol, glycerin and their mixtures have been carried out more detail.Results of investigation of influence of ethanol content in reaction solution at different ratios of initial reagents to one mole of TEOS have shown that with increasing of ethanol content the time of fluidity loss has increased and consequently the time of gel-formation also has increased (Figure 2).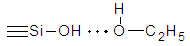 owing to which processes of polycondensation and gel-formation are slow down.
owing to which processes of polycondensation and gel-formation are slow down.4. Conclusions
- The nanocomposite material was obtained by the one-pot sol-gel method on the basis of polycaproamide and silica. The transition process of nanocomposite material from a liquid state (sol) to a solid (gel) was studied resulting in the formation of 3-dimensional net inside polycaproamide.It is determined that the gelation kinetics depends on the ratio of the main components of the reaction system of TEOS: PCA: HClL.The alcohols (ethanol or glycerol) alone or their mixture in different ratios have a significant influence on the gelation time. It is shown that the addition of ethanol to the reaction mixture slows down the process of gelling.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML