-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2015; 5(5): 106-116
doi:10.5923/j.ijmc.20150505.02

Novel Antimicrobial Polymer Based on Hydantoin
Ahmed K. Eid1, EL-Refaie S. Kenawy1, Ahmed A. El-Barbary2
1Department of Chemistry, Polymer Research Group, Faculty of Science, Tanta University, Tanta, Egypt
2Department of Chemistry, Faculty of Science, Tanta University, Tanta, Egypt
Correspondence to: Ahmed K. Eid, Department of Chemistry, Polymer Research Group, Faculty of Science, Tanta University, Tanta, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Infections by microorganisms, such as gram-positive and gram-negative bacteria, virus, fungi, and protozoa are major concerns in clinical and pharmaceutical areas (drugs, medical devices, and food industry (food packaging, storage, and fresh products)). New antimicrobial polymers were prepared by the reaction of 5,5-dimethylimidazolidine-2,4-dione, 5,5-diphenylimidazolidine-2,4-dione, 1-(2,5-dioxoimidazolidin-4-yl)urea, 5,5-diphenyl-2-thioxoimidazolidin-4-one and 5-benzylidene-imidazolidine-2,4-dione with 6-O-Tosyl-N-phthaloyl chitosan and reaction of 5,5-dimethylimidazolidine-2,4-dione, 5,5-diphenyl-2-thioxoimidazolidin-4-one and 5-benzylidene-imidazolidine-2,4-dione with O-succinoyl chitosan chloride. The reaction achieved by activation of hydroxyl groups on the C-6 position of glucosamine residues of chitosan. The structure of the resulting materials was characterized with Fourier transform infrared-spectroscopy, X-ray diffraction TGA analysis and transmission electron microscope. Antimicrobial activity of the polymers was studied against Gram-negative bacteria, Gram-positive bacteria by well diffusion method and cut plug method. Polymers showed good or moderate antimicrobial activities.
Keywords: Chitosan derivatives, Imidiazolidine-2,4-diones, Antimicrobial activity
Cite this paper: Ahmed K. Eid, EL-Refaie S. Kenawy, Ahmed A. El-Barbary, Novel Antimicrobial Polymer Based on Hydantoin, International Journal of Materials and Chemistry, Vol. 5 No. 5, 2015, pp. 106-116. doi: 10.5923/j.ijmc.20150505.02.
Article Outline
1. Introduction
- Infections by microorganisms, such as gram-positive and gram-negative bacteria, virus, fungi, and protozoa are major concerns in clinical and pharmaceutical areas (drugs, medical devices, and food industry (food packaging, storage, and fresh products)). The diseases caused by these microorganisms provoke serious health problems that in severe cases lead to death. Diseases related to the proliferation of microorganisms are particularly significant in hospitals where the risk of infection by microorganisms is a major concern, mainly when complicated surgical procedures are conducted. However, illnesses caused by poor personal hygiene and rotten or contaminated food should also be considered an important issue [1–3]. Therefore, the development of materials that exhibit antimicrobial activity appears to be highly relevant in health care. According to Musumeci et al. [4] an antimicrobial agent is a substance that kills or inhibits the development and the multiplication of microorganisms, such as bacteria, fungi, protozoa or viruses. Among numerous materials having this feature, chitosan and its derivatives can be highlighted. In what follows, some results related to the bacterial activity of chitosan and chitosan-derivatives are presented.Chitosan is a partially deacetylated derivative of chitin, consisting of β-(1,4)-2-amino-2-deoxy-D-glucopyranose and small amounts of N-acetyl-D-glucosamine [5]. Chitosan-derivatives are usually obtained by chemical modification of the amino or hydroxyl (especially at C6 position in the chitosan backbone) groups of chitosan for improving the physicochemical properties [5, 6]. Chitosan and chitosan-derivatives have been extensively used to obtain polyelectrolyte complexes, due to their polycationic nature and their biological properties (biodegradability, biocompatibility, low toxicity, mucoadhesivity and antimicrobial) [7–9]. Imidazolidine-2,4-diones belong to significant heterocyclic compounds, these Imidazolidine-2,4-diones have many applications as antitumor, antiarrhythmic, anticonvulsant, herbicidal and others. Aplysinopsins isolated from marine organisms [10] are examples of imidazolidine-2,4-diones containing natural products exhibiting cytotoxicity towards cancer cells and the ability to affect neurotransmitters. In this study new microbicidal polymers were prepared by the reaction of 5,5-dimethylimidazolidine-2,4-dione I,5,5-diphenylimidazolidine-2,4-dione II, 1-(2,5-dioxoimidazolidin-4-yl)urea III, 5,5-diphenyl-2-thioxoimidazolidin-4-one VI [11] and 5-benzylidene-imidazolidine-2,4-dione V [11] with 6-O-Tosyl-N-phthaloyl chitosan and reaction of 5,5-dimethylimidazolidine-2,4-dione,5,5-diphenyl-2-thioxoimidazolidin-4-one and 5-benzylidene-imidazolidine-2,4-dione with O-succinoyl chitosan chloride. The reaction achieved by activation of hydroxyl groups on the C-6 position of glucosamine residues of chitosan. The structure of the resulting materials was characterized with Fourier transform infrared-spectroscopy, X-ray diffraction, thermo gravimetric analysis, and transmission electron microscope. Antimicrobial activity of the polymers was studied against Gram-negative bacteria, Gram-positive bacteria by well diffusion method and cut plug method. Polymers showed good or moderate antimicrobial activities.
2. Experimental
2.1. Materials
- Chitosan (molecular weight of 300 kDa and 90% of degree of deacetylation) was purchased from Acros Organic, dimethylformamide, 1,2-diphenylethane-1,2-dione, 1,4 dioxane, benzaldehyde, imidazolidine-2,4-dione, 5,5-dimethyl imidazolidine-2,4-dione,5,5-diphenylimidazolidine-2,4-dione, phthalic anhydride, triethylamine, toluene-4-sulfonyl chloride, were purchased from Sigma Aldrich.
2.2. Preparation of 6-O-tosyl-N-phthaloyl chitosan (VI)
- Triethylamine (6.868 g, 0.068 mol) and toluene-4-sulfonyl chloride (3.572 g, 0.0188 mol) dissolved in DMF (20 mL) were gradually added to a cooled to 4–8°C solution of N-phthaloylchitosan [12, 13] (0.54 g, 0.00188 mol) in DMF (50 mL). The mixture was stirred overnight at 8°C. The precipitate obtained by pouring the solution onto ice water, collected by filtration, washed with chloroform and dried to give 0.63 g, yield 83%. FT-IR spectrum (KBr) (cm-1): 1631 cm–1 (-C=N-) 1177 cm–1 due to tosyl Groups and 1578 cm–1 aromatic group, XRD patterns show (Fig. 2) prominent peaks corresponding to the basal spacing of 2Ə = (14.43°, 17.13°).
2.3. O-Succinoyl chitosan chloride (VII)
- SOCl2 (22 ml, 0.03 mol) was added drop wise to O-Succinoyl chitosan [14] (3.89 g, 0.01 mol) (3:1 molar) in anhydrous benzene (20 ml). Reaction mixture was stirred at r.t. for 24 h. The reaction mixture was heated at 60°C for 3 h. residual solid was filtered off. The precipitate formed washed several times with anhydrous benzene then dried under vacuüm to give 2.64 g, (yield 65%). [14] FT-IR spectrum (KBr) (cm-1): band at 1731 cm-1 was attributed to carbonyl group of ester group [C=O of O (COR)] and the peak at 1662 cm-1 was assigned to the carbonyl group of acylamino group the signal of the phthalimide group at 1770, 1710 cm-1.
2.4. Synthesis of 6-O-tosyl-N-phthaloyl chitosan / 5,5-Dimethylimidazolidine-2,4-dione (VIII)
- 5,5-Dimethylimidazolidine-2,4-dione I (0.128g 0.001 mol) was stirred with sodium hydroxide (0.04 g, 0.001 mol) in ethanol for 24 h at 25°C followed by addition 6-O-Tosyl-N-phthaloyl chitosan VI (0.457 g, 0.001 mol) portion wise. The reaction mixture was refluxed for 48 h, cooled. The residual solid was filtered off, the residue was purified by ethanol and dried under vacuum oven at 40°C for 48 h to afford 0.35g, yield 80% , FT-IR spectrum (KBr) (cm-1) (Fig. 1): showed the following bands 3450 (O-H), 3280 (Ν-H amide, CO-NH-CPh2), 3000 (C-H aliphatic), 1771,1736 (C=O, phthalimide) 1711 (C=O amide, NH-CO-CMe), 1640, (C=O amide, NH-CO-N), 1386 (CH2), 1159 (C-O-C), The XRD patterns show (Fig. 2) prominent peaks corresponding to the basal spacing of 2Ə = (4.94°, 14.03°, 17.40°, 18.33°, 23.85°).
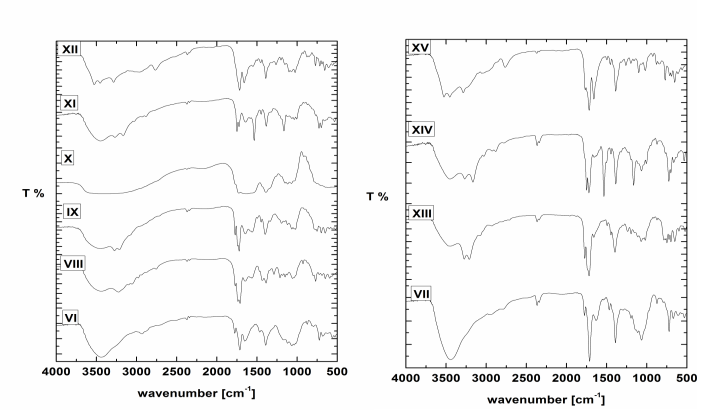 | Figure 1. FT-IR spectra of VI-XV |
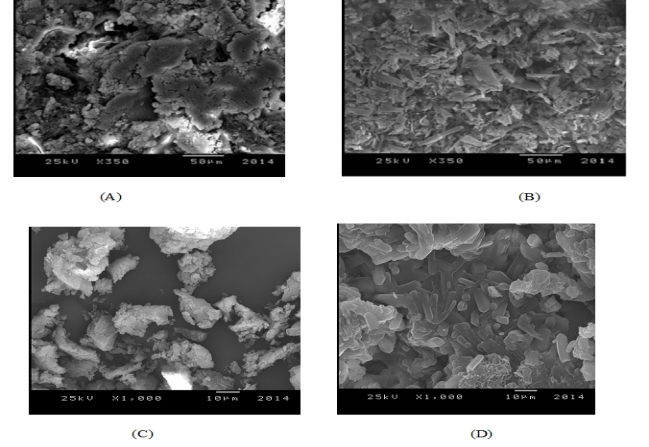 | Figure 2. SEM images of (A) VI, (B) VIII, (C) VII and (D) XIII |
2.5. Synthesis of 6-O-tosyl-N-phthaloyl chitosan / 5,5-diphenylimidazolidine-2,4-dione (IX)
- 5,5-diphenylimidazolidine-2,4-dione II (0.252 g, 0.001 mol) was stirred with sodium hydroxide (0.04 g, 0.001 mol) in ethanol for 24 h at 25°C followed by addition 6-O-Tosyl-N-phthaloyl chitosan VI (0.457 g, 0.001 mol) portion wise. The reaction mixture was refluxed for 48 h, cooled. The residual solid was filtered off, the purification of the residue by ethanol and dried under vacuum oven at 40°C for 48 h to afford 0.41 g, yield 75% , FT-IR spectrum (KBr) (cm-1) (Fig. 1): showed the following bands 3450 (O-H), 3240 (Ν-H amide, CO-NH-CPh2), 3000 (C-H aromatic), 1771,1735 (C=O, phthalimide) 1723 (C=O amide, NH-CO-CPh), 1643, (C=O amide, NH-CO-N)., XRD patterns show (Fig. 2) prominent peaks corresponding to the basal spacing of 2Ə = (4.96°, 11.35°, 13.21°, 16.79°, 17.40°, 20.40°, 22.50°, 26.51°).
2.6. Synthesis of 6-O-tosyl-N-phthaloyl chitosan / 1-(2,5-dioxoimidazolidin-4-yl)urea (X)
- To a suspension of 1-(2,5-dioxoimidazolidin-4-yl)urea III (0.158g, 0.001 mol) in DMF (10 mL), 6-O-Tosyl-N-phthaloyl chitosan VI (0.457 g, 0.001 mol) was added portion wise. The reaction mixture was refluxed for 48 h, cooled. The residual solid was filtered off, the residue was purified by ethanol and dried under vacuum oven at 40°C for 48 h to afford 0.36g, yield 80%, FT-IR spectrum (KBr) (cm-1) (Fig. 1): showed the following bands 1777 cm–1 is characteristic of carbonyl in phthalimide and absorption at 1726, 1629 cm–1 are characteristic of carbonyl in 1-(2,5-dioxoimidazolidin-4-yl)urea, XRD patterns show (Fig. 2) prominent peaks corresponding to the basal spacing of 2Ə = (17.23°, 20.34°, 29.75°).
2.7. Synthesis of 6-O-tosyl-N-phthaloyl chitosan / 5,5-diphenyl-2-thioxoimidazolidin-4-one (XI)
- Into solution of 5,5-diphenyl-2-thioxoimidazolidin-4-one IV (0.26g, 0.001 mol) in DMSO (10 mL), 6-O-Tosyl-N-phthaloyl chitosan VI (0.457 g, 0.001 mol) was added portion wise. The reaction mixture was refluxed for 48 h, cooled. The residual solid was filtered off, the residue was purified by ethanol and dried under vacuum oven at 40°C for 48 h to afford 0.45 g, yield 75%, FT-IR spectrum (KBr) (cm-1) (Fig. 1): showed the following bands 3450 (O-H), 3200, (N-H), 3070 (C-H aromatic), 1752, 1725, are characteristic of carbonyl in phthalimide and absorption at 1637, (C=O) cm–1 is characteristic of carbonyl in 5,5-diphenyl-2-thioxoimidazolidin-4-one and at 1180 (C-S), XRD patterns show (Fig. 2) prominent peaks corresponding to the basal spacing of 2Ə = (11.50°, 14.74°, °, 17.70°, 21.45°, 23.96°).
2.8. Synthesis of 6-O-tosyl-N-phthaloyl chitosan / 5-benzylidene-imidazolidine-2,4-dione (XII)
- 5-benzylidene-imidazolidine-2,4-dione IV (0.188g 0.001 mol) was stirred with sodium hydroxide (0.04 g, 0.001 mol) in ethanol for 24 h at 25○C followed by addition 6-O-Tosyl-N-phthaloyl chitosan VI (0.457 g, 0.001 mol) was added portion wise. The reaction mixture was refluxed for 48 h, cooled. The residual solid was filtered off, the purification of the residue ethanol and dried under vacuum oven at 40°C for 48 h to afford 0.40 g, yield 75% , FT-IR spectrum (KBr) (cm-1) (Fig. 1): showed the following bands 3550,(O-H), 3070 (C-H aromatic), 1715 broad band are characteristic of carbonyl in phthalimide 1658, broad band are characteristic of carbonyl in 5-benzylidene- imidazolidine-2,4-dione., XRD patterns show (Fig. 2) prominent peaks corresponding to the basal spacing of 2Ə= (7.79°, 15.70°, 17.74°, 26.60°).
2.9. Synthesis of O-succinoyl chitosan / 5,5-diphenylimidazolidine-2,4-dione (XIII)
- To a suspension of 5,5-diphenylimidazolidine-2,4-dione II (0.252 0.001 mol) with O-succinoyl chitosan chloride VII (0.407g 0.001 mol) in 1,4 dioxane (10 ml), pyridine (0.79g, 0.001 mol) was added drop wise. The reaction mixture was stirred at r.t. for 72 h. The mixture was reflux 48 h then cooled, The residual solid was filtered off washed with 1,4 dioxane, dried under vacuüm oven at 40°C for 48 h to afford 0.40 g 65% yield. FTIR (KBr) (cm-1): 3450 (O-H), 3210 is characteristic of (N-H) in 5,5-diphenylimidazolidine-2,4-dione, 3070 (C-H aromatic), 1773 is characteristic of carbonyl in 5,5-diphenylimidazolidine-2,4-dione, 1719, 1660, broad bands are characteristic of carbonyl in phthalimide (C=O), 1400 (CH2), 1023 (C-O-C).
2.10. Synthesis of O-succinoyl chitosan / 5,5-diphenyl-2-thioxoimidazolidin-4-one (XIV)
- To a suspension of 5,5-diphenyl-2-thioxoimidazolidin-4-one IV (0.26g 0.001 mol) with O-succinoyl chitosan chloride VII (0.407g 0.001 mol) in 1,4 dioxane (10 ml), pyridine (0.79 g, 0.001 mol) was added drop wise. The reaction mixture was stirred at r.t. for 72 h. The mixture was reflux 48 h then cooled, The residual solid was filtered off washed with 1,4-dioxane dried under vacuüm oven at 40°C for 48 h to afford 0.44g, 70% yield. FTIR (KBr) (cm-1): 3436 (O-H), 3274 is characteristic of (N-H) in 5,5-diphenylimidazolidine-2,4-dione, 3050 (C-H aromatic), 1764 is characteristic of carbonyl in 5,5-diphenylimidazolidine-2,4-dione, 1713, 1645 broad bands are characteristic of carbonyl in phthalimide (C=O), 1390 (CH2), 1030 (C-O-C).
2.11. Synthesis of O-succinoyl chitosan / 5-benzylidene imidazolidine-2,4-dione (XV)
- To a suspension of 5-benzylideneimidazolidine-2,4-dione V (0.188g 0.001 mol) with O-succinoyl chitosan chloride VII (0.407g 0.001 mol) in 1,4-dioxane (10 ml), pyridine (0.7 g, 0.001 mol) was added drop wise. The reaction mixture was stirred at r.t. for 72 h. The mixture was reflux 48 h then cooled, The residual solid was filtered off, washed with 1,4-dioxane dried under vacuüm oven at 40°C for 48 h to afford 0.42 g 76% yield. FTIR (KBr) (cm-1): 3561, 3453, 3267 (O-H), (N-H), 3030 (C-H aromatic), 3150 (C-H aliphatic), 1760 band is characteristic of carbonyl in 5-benzylideneimidazolidine-2, 4-dione, 1715, 1660 broad bands are characteristic of carbonyl in phthalimide (C=O), 1380 (CH2), 1070 (C-O-C).
2.12. Characterization
- FT-IR spectra were recorded on Bruker, Tensor 27 FT-IR spectro-photomer with frequency range 4000 cm-1 to 400 cm-1 (Central Lab, Tanta University, Egypt) with KBr pellets. Scanning Electron Microscope – JEOL JSM 5200 LV (electron microscope unit, Faculty of Medicine, Tanta University, Egypt). The XRD analysis was measured by GRN, APD 2000 PRO X-Ray diffraction, using Cu Ka (1.54 Ao) at 40 Ku (Central Lab. Tanta University, Egypt), TGA data were obtained by using Shimadzu thermal analyzer system at a heating rate of 10 °C/min, sample weight of 5-6 mg, under nitrogen (20 ml/min) flow. The range investigated from 25°C- 800°C. TGA-50 furnace with a M3 microbalance, and TA72 Graphware software were employed for thermal analyses (Central Lab. Tanta University, Egypt).
3. Biological Activities of the Modified Polymers
3.1. Tested Microorganisms
- The microbial strains were obtained from Bacteriological lab, Microbiology section, Botany Department, Faculty of Science, Tanta University: The Gram-negative bacteria (Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 9027), Gram-positive bacteria (Staphylococcus aureus ATCC 6633, Staphylococcus epidermidis ATCC 12228), fungi (Candida albicans and Aspergillus niger) were used to examine the antimicrobial activity of the polymers The bacterial strains were maintained on nutrient agar (3 g peptone, 5 g NaCl, 5 g beef extract, 20 g agar per liter, pH=7.4).
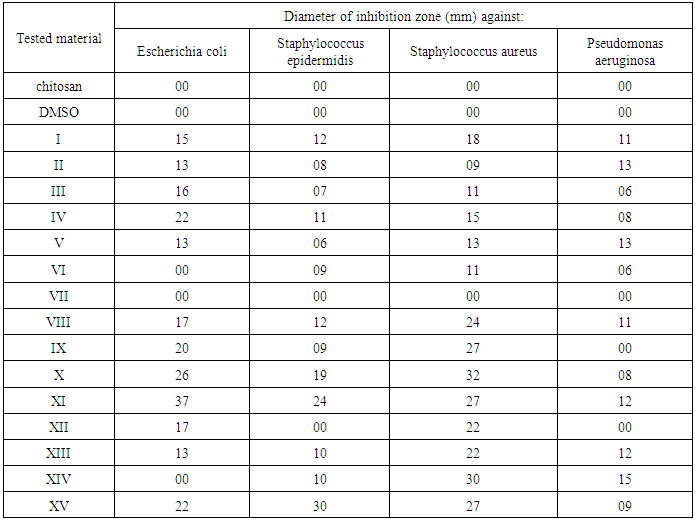
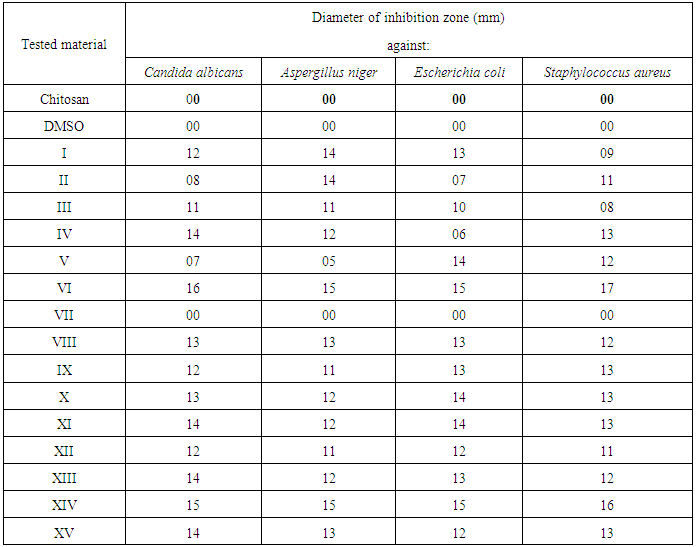
3.2. Well Diffusion Method for Screening of Antimicrobial Activity for Tested Complexes
- The antimicrobial activity of the tested samples was determined by well diffusion method [15] Powder test samples (20 mg/ml) were dissolved in DMSO. In case of nanocomposites the samples were dispersed in DMSO. Wells were then created and 50 μl of the samples were pipetted into each well to detect the most sensitive microorganisms against investigated polymers. DMSO was used as negative control. The plates were incubated at 37°C for bacteria were examined for inhibition zones development. A caliper was used to measure the inhibition zones. Three replicates were carried out at least. The inoculum's concentrations were 6.5x 105 CFU for bacteria. After incubation for 24 h for bacteria, yeasts and at 48 h for fungi, the agar plates were checked for the diameter of inhibition zone. The reaction of the microorganisms with antimicrobial polymers was determined by the size of the inhibitory zone.
3.3. Cut Plug Method for Screening of Antimicrobial Activity for Tested Complexes
- Cut plug method was employed to determine the antimicrobial activity of the chosen products as follows: Freshly prepared spore suspension of different test microorganisms (0.5 ml of about 106 cells/ml) was mixed with 9.5 ml of melting sterile Sabouraud's dextrose medium (for fungi) or nutrient agar medium (for bacteria) at 45°C, poured on sterile Petri dishes, and left to solidify at r.t. Regular wells were made in the inoculated agar plates by a sterile cork borer of 0.70 mm diameter. Each well was filled with 20 mg of each tested powder. Three replicas were made for each test, and all plates were incubated at 27°C for 3 days for fungi, and at 32°C for 24 h for bacteria. Then the average diameters of inhibition zones were recorded in centimeters, and compared for all plates. [16]
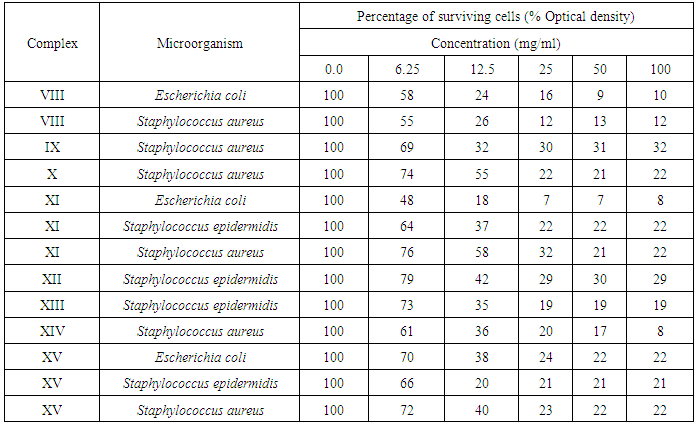
3.4. Minimal Inhibitory Concentrations (MICs)
- The most antimicrobial active compounds were chosen to determine their MIC values against the most sensitive microorganism by tube dilution assay.
3.5. Tube Dilution Assay
- Nutrient broth was used for the tube dilution to determine MICs of tested bacteria. The MIC value of tested compounds was determined using two-fold broth micro-dilution. One millimeter of sterile media was added to 1 ml of different compound concentrations (100, 50, 25, 12.5, 6.3, 3.2 and 1.6, 0.8, 0.4 and 0.2 mg/1 ml). The tubes were then inoculated with a drop of microbial suspension (25 μl) and incubated at 37°C for 24 h. Tetracycline (100-0.0031 mg/ml) was used as standards antimicrobial drug (positive control). DMSO was used as the negative control. After incubation, certain amount of each tube was spreeded on agar plates and then incubated for 24 h, and then the numbers of colonies were counted. The MIC endpoints were detected as the lowest concentration of tested compounds at which the growth of the tested microorganisms was inhibited. The minimum bactericidal concentration (MBC) endpoints are defined as the lowest concentration of antimicrobial agent that kills > 99.9% of the initialbacterial population and detected when no visible growth of bacteria was observed on the agar plates. [17]
3.6. Statistical Analysis
- All experiments and analytical determinations were replicated at least three times and mean value was calculated.
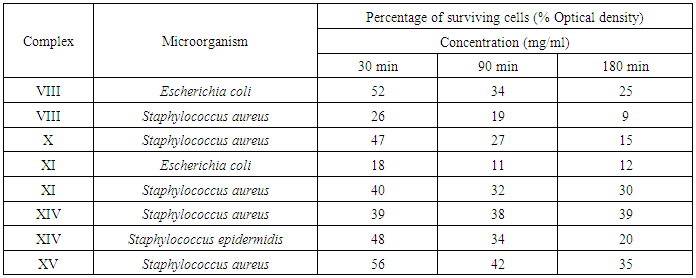
3.7. Uses of the most Efficient Antimicrobial Polymers Water Treatment
- Fixed quantity of the complexes (50 mg) were placed into different column for purifications certain amount of previously prepared infected water (5 ml of about 106 cells/ml) with some microorganism ((Escherichia coli ATCC 8739, Staphylococcus aureus ATCC 6633, Staphylococcus epidermidis ATCC 12228)). The inhibition of the complexes were taken throw different times (30 min, 90 min and 180 min) in order to establish the best result and Percentage of surviving cells was recorded for each tested material.
4. Results and Discussion
- In this work, A series of polymers containing chemically bound imidazolidine-2,4-dione have been prepared by reaction of 5,5-dimethylimidazolidine-2,4-dione I, 5,5-diphenylimidazolidine-2,4-dione II, 1-(2,5-dioxoimidazolidin-4-yl)urea III, 5,5-diphenyl-2-thioxoimidazolidin-4-one VI and 5-benzylidene-imidazolidine-2,4-dione V with 6-O-Tosyl-N-phthaloyl chitosan. Modification on the C6 of chitosan was carried out by the nucleophilic replacement of the (tosyl) group with N in imidazolidine-2,4-dione or S 2-thioxoimidazolidin-4-one.
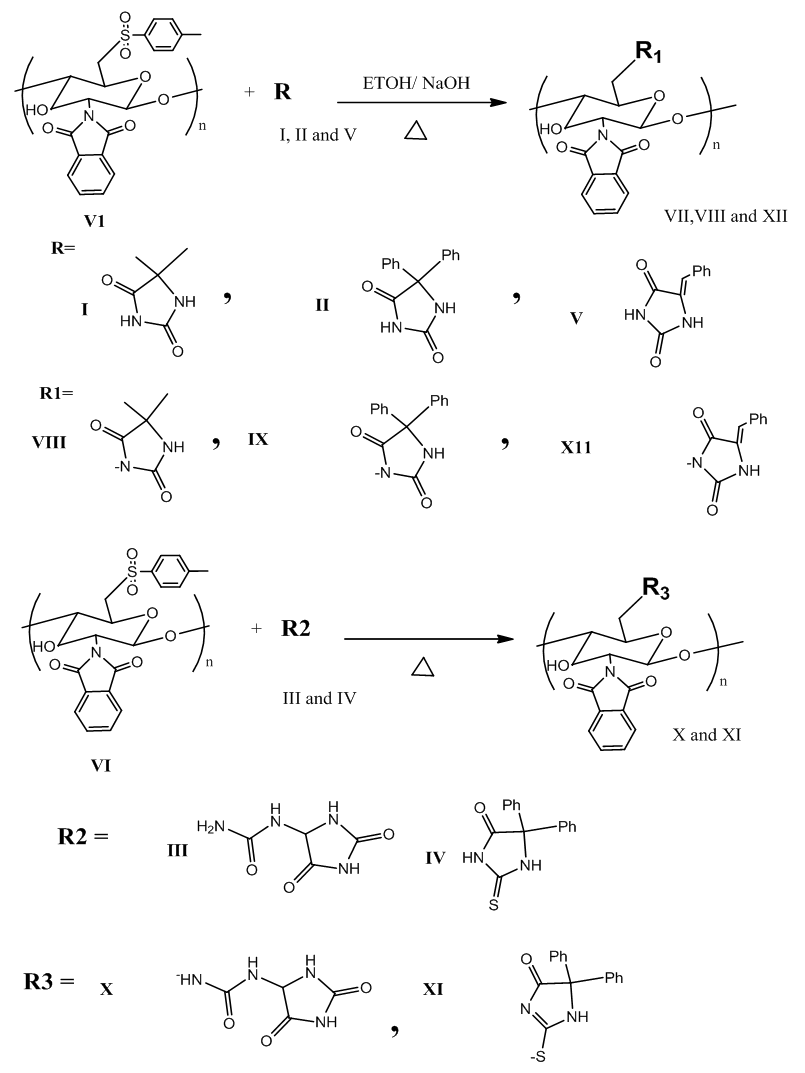 | Scheme 1. Modification of (VI) with (I-V) |
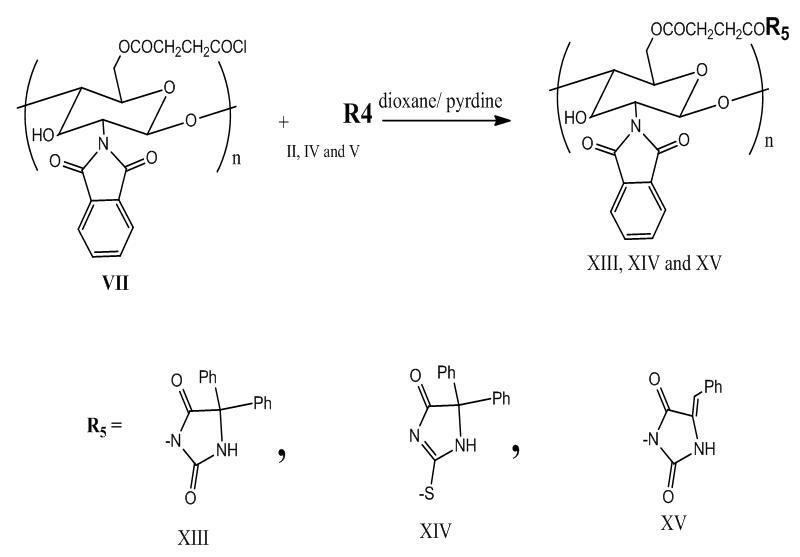 | Scheme 2. Modification of (VII) with (II, IV and V) |
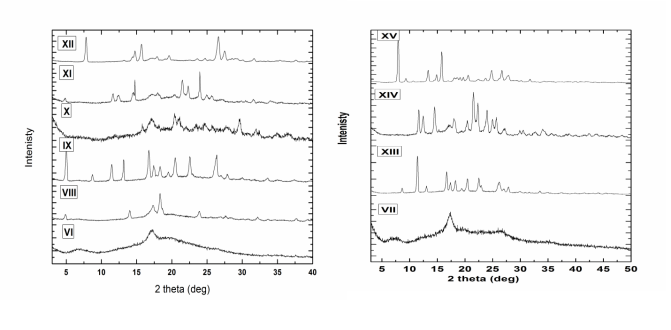 | Figure 3. X-Ray spectra of VI - XV |
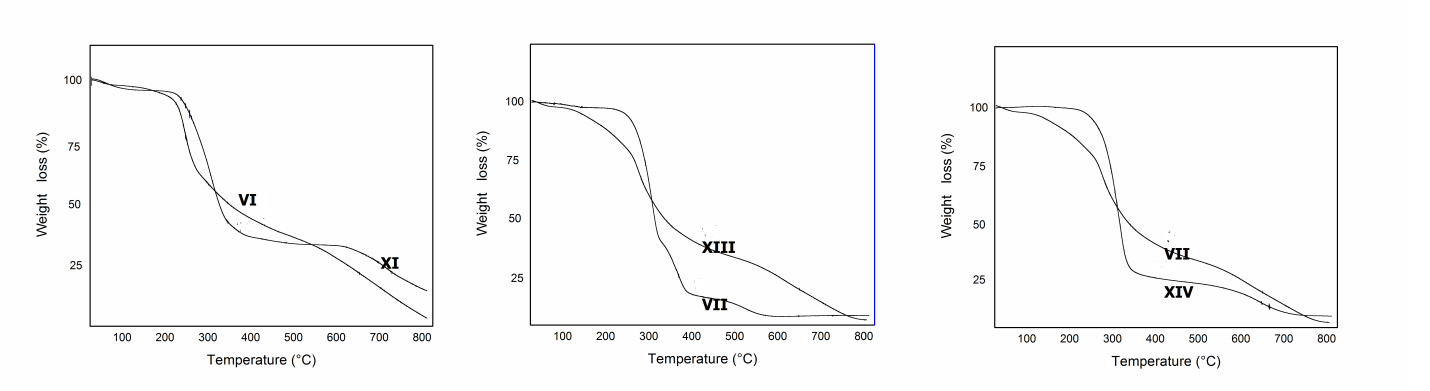 | Figure 4. TGA analysis of VI, VII, IX, XIII and XIV |
|
|
|
|
5. Conclusions
- Modification of some imidiazoldin-2,4-dione derivatives on the C-6 position of glucosamine residues of chitosan was achieved by protecting amino functionality by phthaloylation and “activating” primary hydroxyl groups of chitosan. Modification of chitosan with different imidiazolidine-2,4-dione derivatives VIII–XV show large improvement of the biological activity against the selected bacteria while compound VI and VII show pour effect. Anti-microbial activity of compound VIII–XV are more effective against Gram-positive than Gram-negative because the more complex architecture of the cell envelope in Gram-negative bacteria, which includes an outer and inner membrane with the periplasmic space in between.
ACKNOWLEDGMENTS
- The authors acknowledge higher studies and research sector at Tanta University for their financial support for project with code number 01-13-03.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML