-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2015; 5(5): 101-105
doi:10.5923/j.ijmc.20150505.01
A Study on Waterborne Polyurethane Coated Polyamide 11 Fiber and Composite Fiber with Nanodiamond
Ayesha Kausar
Nanosciences and Catalysis Division, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanosciences and Catalysis Division, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
In this paper, the waterborne polyurethane (WPU) coating was prepared using polyaddition reaction of polymethylene glycol, 2,2-bis(hydroxymethyl) propionic acid, and hexamethylene diisocyanate. The polyamide 11 (PA11) and PA11/nanodiamond (ND) fibers were prepared using simple method. The dip-coating technique was used to coat the fibers with WPU. The fractured surface of WPU coated fiber containing ND was smooth according to scanning electron microscopic images; however the morphology of the fibers with out nanodiamond was less smooth and adjoined at various places. The effect of pressure on the adhesive strength of WPU adhesive containing fibers with various ND content was also studied. The adhesive strength was continuously increased with the increase of pressure till 25 kg f/cm2 and also with nanodiamond loading. The water absorbing tendency of WPU coated polyamide fibers enhanced from 5.4-7.8 % with filler loading.
Keywords: Water borne polyurethane, Polyamide 11, Fiber, Nanodiamond, Adhesive strength
Cite this paper: Ayesha Kausar, A Study on Waterborne Polyurethane Coated Polyamide 11 Fiber and Composite Fiber with Nanodiamond, International Journal of Materials and Chemistry, Vol. 5 No. 5, 2015, pp. 101-105. doi: 10.5923/j.ijmc.20150505.01.
Article Outline
1. Introduction
- Polyurethane is one of the most attractive man-made elastomers. Due to its distinctive features, more consideration has been attracted to the morphology, synthesis, chemical and mechanical properties [1, 2]. The hard-segment is comprised of a low-molecular weight diol or diamine reacted with diisocyanate while the soft-segment is normally polyester or polyether macrogel of molecular weight between 1000 and 3000. Due to the difference in the chemical structure, microdomains are formed by hard and soft segment by mutual attractions relating intermolecular hydrogen bonding [3]. As compared to solvent based polyurethane, waterborne polyurethane (WPU), is of several important merits, such as having low content of volatile organic compounds, being environmentally benign, having special physical and chemical efficiency, and so on. Thus, it has been extensively used in adhesives, inks, coatings, and other fields. However, to the best of our knowledge, the waterborne PU typically suffers from some imperfect properties, comprising hardness, heat resistance, water and solvent resistance. For waterborne polyurethane systems, during the drying process only water was evaporated, thus representing these systems safe with regard to the environment. They are non-flammable, non-toxic and do not produce polluted air or wastewater [4, 5]. The waterborne polyurethane generation has been paying attention much more due to environmental consideration. The synthesis of WPU is free from emission of organic volatile solvent; as a result the atmosphere is protected from pollution. Besides, WPU has received significant interest in the past few decades due to its remarkable usefulness in coatings and adhesives for various substrates [6]. Their application comprises areas such as construction, packing, automotive, electronics, transportation, tape, textiles, paper, and footwear [7, 8]. One important feature of polyurethane ionomers is their ability to diffuse or dissolve in water by incorporation of significant amount of ionic moiety, which is a great advantage over conventional solvent borne polyurethane [9]. It is well predictable that for the development of a stable polyurethane ionomer, a minimum ionic content is required depending on the type of the ionic species. In addition, the interface between ions and their counter ions is dependable for the effect on their properties. On the properties of an ionomer, the degree of neutralization and content of ionic component has very considerable effect [10]. In recent years, in modifying the formation of WPU, hybrids of WPU and nanofiller play an essential role. In comparison to pure WPU, the nanocomposite could not only bring great enhancement in thermostability, mechanical property, and water resistance, but some special properties of nanomaterials were also provided, such as thermal insulation, ultraviolet shielding, abrasive resistance, conductivity, and other functions [11, 12]. Traditionally, the WPU composites were prepared by sol-gel, in-situ; or direct mixing processes [13-15]. Polyamide fibers have a large distribution of the worldwide market of synthetic fibers. Major distinctiveness of polyamide fibers are their hydrophobicity and low reactivity with most common chemical agents [16]. The high hydrophobicity makes these fibers less suitable to be used in the manufacture of garments that are in contact with human skin. The fiber performance during textile handing out is determined by the orientation grade of polymeric chains, the high crystallinity, and the concentration of amino group in terminal positions [17]. The polar groups are present in rigid polyamide segments which can form intermolecular hydrogen bond. The intermolecular hydrogen bond makes the molecular chains set orderly to form rigid domain, which improves the polymer’s tensile strength and thermal resistance. Researchers have also transformed the thermal and physical properties of polymer fibers by composite system manufacture. Nanodiamond (ND) produced by detonation production are based on uniform and small particles of ~5 nm in diameter and signifies an available and huge surface area. Bulky diamond has several matchless distinctiveness comprising superior thermal conductivity, stiffness, hardness, Young’s modulus, high refraction index, and a high resistivity [18]. There are frequent research efforts addressing the thermoplastics strengthening with ND. Even without surface modification and at low concentrations, ND has been accounted to support several thermoplastics [19, 20]. In this effort, PA11 and nanodiamond-based fibers have been prepared and coated with WPU. The structure, morphological, thermal, and water absorption characteristics of novel fibers have been investigated.
2. Experimental
2.1. Materials
- Polymethylene glycol (PTMG, number-average molecular weight=2000 g/mol), 2,2-bis(hydroxymethyl) propionic acid (DMPA, 98%), N-methyl-2-pyrrolidone (NMP, 98%), polyamide 11 (3 mm, pellets), hexamethylene diisocyanate (HDI, ≥98.0%), and diamond nanopowder (<10 nm particle size) were supplied by Aldrich.
2.2. Characterization Techniques
- IR spectra were taken at room temperature with a resolution of 4 cm-1 using Excalibur Series FTIR Spectrometer, Model No. FTSW 300 MX manufactured by BIO-RAD. The scanning electron microscopic (SEM) images were obtained by Scanning Electron Microscope S-4700 (Japan Hitachi Co. Ltd.). The adhesion property was measured by ASTM D 1876-01 using United Data System tension meter (T-peel test). Water absorption tendency of the fibers was evaluated by immersing equal weights of samples in water for 100 h at ambient temperature. The water uptake was calculated using Eq 1.
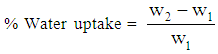 | (1) |
2.3. Preparation of Polyamide 11 (PA11) Fibers
- The polyamide 11 was placed in a vacuum oven at 60°C for 24 h to remove any moisture content. The neat PA11 fibers were prepared using a Brabender single screw extruder (Intelli-torque) and a single hole fiber die (diameter = 0.012 inches L/D ratio = 3). The temperature was kept at 200°C with a screw speed of 3 rpm. The extruded fibers were stretched with draw ratio 3 using Instron fiber clamps at room temperature [21].
2.4. Preparation of Polyamide 11/nanodiamond (PA11/ND) Fibers
- The polyamide 11 and nanodiamond were placed in a vacuum oven at 70°C for 24 h to remove any moisture content. The PA11 and nanodiamond nanocomposite fibers were also prepared using a Brabender single screw extruder with a single hole fiber die. The temperature was kept at 200°C with a screw speed of 3 rpm. The extruded fibers were stretched with draw ratio 3 using Instron fiber clamps at room temperature. FTIR (cm-1): 3345 (N–H stretching vibration), 2999 (C–H stretching vibration), 1670 (C=O), 1599 (N–H bending vibration).
2.5. Formation of Waterborne Polyurethane Coating (WPU)
- The waterborne polyurethane was prepared with the prepolymer mixing process [22]. The PTMG was placed in a three-necked flask equipped with a stirrer, thermometer, condenser, inlet of dry nitrogen, and a heat jacket. DMPA in NMP (1:1 w/w) was added to the flask, and the mixture was refluxed at 60°C. Then, HDI was added to the flask, and the mixture was heated to 80°C (Fig. 1).
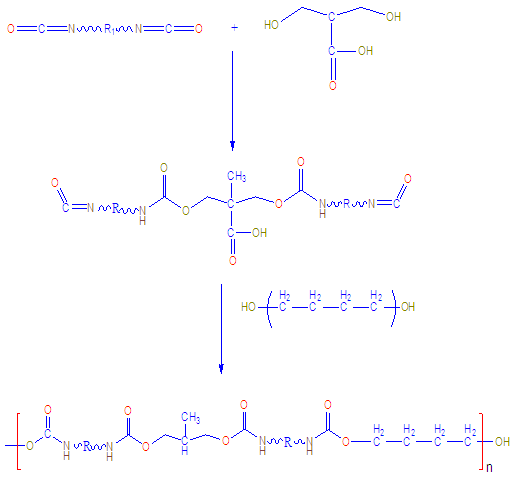 | Figure 1. Formation of WPU |
2.6. Preparation of Waterborne Polyurethane Coated Fibers (WPU/PA11 and WPU/PA11/ND)
- The fibers were coated with above prepared mixture using simple dip-coating technique with a dipping rate of 100 mm min-1. The immersion time was kept 6h for all the fibers. The number of deposited layers was 1, 5, or 10 [23]. After deposition, the samples were washed and dried at 80°C for 24 h (Fig. 2). FTIR (cm-1): 3250 cm-1 (N–H stretch), 1596 cm-1 (N–H bend), 3041 cm-1 (aromatic C–H stretch), 2270 cm-1 (–N=C=O stretch), 1711 cm-1 (urethane C=O stretch), 1660 cm-1 (amide C=O stretch), 1240 cm-1 (C–O stretch).
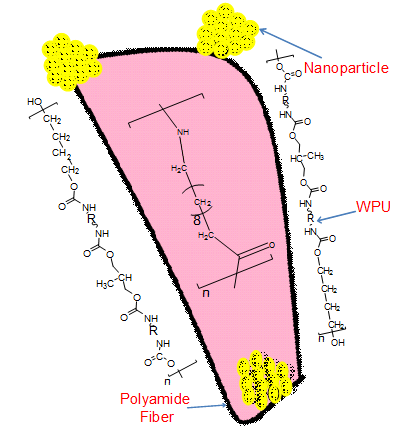 | Figure 2. A schematic diagram for WPU/PA11/ND fiber |
3. Results and Discussion
3.1. Microscopic Study
- The morphology of the tested specimens was examined using SEM. The micrographs of WPU/PA11 showed some what even surface but the fibers produced were not aligned and had aggregation and joined surface at several places (Fig. 3A). However no traces of unevenly dispersed polyurethane were found on the fiber surface due to hydrogen bonding between the WPU and polyamide. The addition of nanodiamonds altered the fiber morphology. The WPU/PA11/ND 1 fibers had very smooth surface without any aggregation or particulate matter (Fig. 3B). As it can be clearly seen that there is uniform alignment of the fibers prepared. The WPU/PA11/ND 5 fibers were also uniformly aligned with smooth surface (Fig. 3C). However there was increase in the diameter of the fibers with the addition of nanodiamond. No fiber pull out phenomenon was observed on the fractured surface showing increase in fiber adhesion. Due to better adhesion of WPU fibers no interface de-bonding or cracking was observed. The morphology study indicated the increase in the strength of the composites.
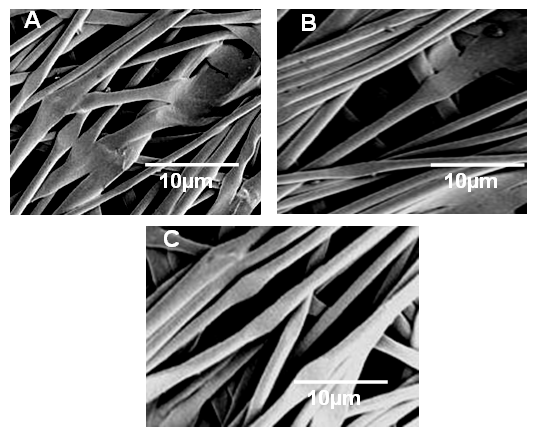 | Figure 3. FESEM images of (A) WPU/PA11; (B) WPU/PA11/ND 1; and (C) WPU/PA11/ND 5 |
3.2. Measurement of Adhesion Strength
- Fig. 4 shows the effect of pressure of adhesion process on the adhesive strength of WPU adhesives containing WPU/PA11/ND 1, WPU/PA11/ND 5 and WPU/PA11/ND 10. The optimal pressing temperature was fixed for each sample and pressure was changed during adhesive process. The adhesive strength was continuously increased with the increased of pressure till 25 kg f/cm2. Second observation was that the WPU/PA11/ND 1 fiber showed lower trend than WPU/PA11/ND 5. Similarly WPU/PA11/ND 10 depicted highest values of adhesion strength with a continuously increasing trend. Therefore the role of ND in increasing the adhesive strength of the composites was obvious. The results suggested that the optimum pressure of WPU adhesives containing fibers was 25 kg f/cm2 and 10 wt. % ND.
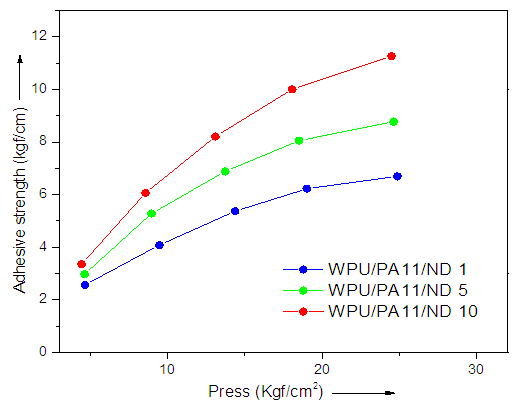 | Figure 4. Effect of pressure on adhesive strength of WPU/PA11/ND |
3.3. Water Absorption Behavior
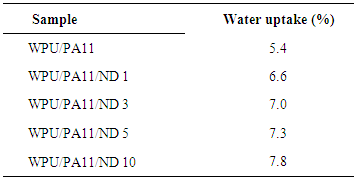
- Table 1 reports the water uptake characteristics of WPU/PA11, WPU/PA11/ND 1, WPU/PA11/ND 3, WPU/PA11/ND 5, and WPU/PA11/ND 10 composite fibers. Fig. 5 shows the relative water uptake characteristics of the fibers. It was observed that the WPU/PA11 1 had water uptake of 6.6% that was higher than the non-coated fiber WPU/PA11 (5.4%). The polyurethane coating as well as the inclusion of nanodiamond in the fibers renders them more hydrophilic. The WPU coated fibers had higher water absorbing tendency than non-coated WPU/PA11. The water uptake values were increased from 6.6 to 7.8%. Consequently, there was 15.4 % increase in water absorption with increase in filler loading [24, 25]. This increase was might be due to the formation of hydrogen bonding linked polyamide-polyurethane linked network.
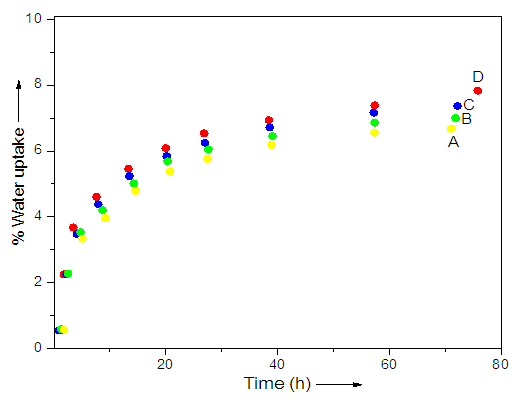 | Figure 5. Water uptake characteristics of (A) WPU/PA11/ND 1; (B) WPU/PA11/ND 3; (C) WPU/PA11/ND 5; and (D) WPU/PA11/ND 10 |
|
4. Conclusions
- This study focused on the effect of WPU coating as well as nanodiamond addition on the morphology, adhesive strength and water absorption of the WPU/PA11 and WPU/PA11 1-10 fibers. The morphology study revealed even and consistently aligned fiber formation for nanodiamond containing samples. The WPU/PA11 10 fibers showed better adhesive strength as well as water absorption characteristics. All the adhesive-bonded samples showed a consciously increasing trend in strength at optimum pressure of 25 kg f/cm2. The WPU coated fibers also had greater water absorption compared with the non-coated polyamide fibers. The WPU coated PA11 fibers showed remarkable physical properties consequently so may act as potential contender for a range of technical applications.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML