-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2015; 5(4): 96-100
doi:10.5923/j.ijmc.20150504.03
Preparation and Characterization of Nylon 6 and Lignin Coated Carbon Nanotube Composite
Ayesha Kausar
Nanosciences and Catalysis Division, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanosciences and Catalysis Division, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Novel lignin modified carbon nanotube (L-CNT) were prepared as reinforcement of nylon 6 composites. The carbon nanotube was coated with lignin using dioxane solvent. The filler was loaded in polyamide-6 composites through in-situ polymerization. Two parameters were varied i.e. (i) sonication time during in-situ reaction and (ii) L-CNT content. The effect of L-CNT on molecular weight (Mn and Mw), glass transition temperature (Tg) and tensile modulus of the composites was studied. The molecular weight of in-situ polymerized PA6 was decreased with the addition of L-CNT content. The same effect was observed with the increase in sonication time due to hindrance in chain growth by L-CNT. Significant increase in the mechanical properties was observed up to the sonication time of 0.5 h. The tensile modulus of the composites was increased to 5.9 GPa in PA 6/L-CNT 10 nanocomposite. However there was 61% increase in the tensile modulus with 10 wt. % nanofiller addition compared with 1 wt. % L-CNT. The glass transition temperature for PA 6/L-CNT 1, PA 6/L-CNT 3, PA 6/L-CNT 5, and PA 6/L-CNT 10 was found as 80, 82, 84, and 101°C at 0.5 h sonication time. Hence there was 21% increase in the Tg upon the addition of 10 wt. % filler compared with 1 wt. % L-CNT addition.
Keywords: Polyamide, Lignin, in-situ polymerization, Nanocomposite, Tg, Tensile modulus
Cite this paper: Ayesha Kausar, Preparation and Characterization of Nylon 6 and Lignin Coated Carbon Nanotube Composite, International Journal of Materials and Chemistry, Vol. 5 No. 4, 2015, pp. 96-100. doi: 10.5923/j.ijmc.20150504.03.
Article Outline
1. Introduction
- Over the past years, discovery of carbon nanotube (CNT) have concerned huge interest owing to their remarkable electrical, physical, and mechanical properties, which make them ideal candidates for various applications in the fields of electronics, nanomechanics, and sensor development [1, 2]. CNT have been categorized into two different categories, i.e. single-walled carbon nanotube (SWCNT) and multi-walled carbon nanotube (MWCNT). The difference between single-walled and multi-walled carbon nanotube is that SWCNT show single-layer of graphene sheet with simple geometry (diameter ranges from 0.3-0.4 nm) whereas MWCNT consist of several layers of graphene sheets. Due to their outstanding characteristic features, CNT can also be used in several fields including mechanical, chemical, and electrical relevance. The CNT-based composites are even more fascinating materials since properties of their constituents are often changed and tuned by a synergistic effect. The polymer/CNT composites are the most representative examples of such hybrid materials [3]. The term lignin is used to illustrate both naturally occurring binding cellulose fibers, biopolymers together in plant cells, and a byproduct from paper/pulp industry, which in fact is a diversity of chemical derivatives characterized by lower molecular weight, changed chemical structure and solubility in aqueous and/or organic phases. From the chemical point of view, the parent lignin is an amorphous polyphenolic material arising from an enzyme mediated dehydrogenative polymerization of three phenylpropanoid monomers, coniferyl, coumaryl, and sinapyl alcohol [4, 5]. Next to cellulose, lignin is the second most abundant renewable natural resource which is highly-branched, three-dimensional biopolymer. It comprises of three phenylpropanoid units such as guaiacyl (G), p-hydroxyphenyl (H) and syringyl (S) (Fig. 1). The distinctive network structure as well as the presence of numerous chemical substituents offer special functional features to lignin such as UV-absorption, stabilizing effect [6], biodegradability, reinforcing effect, anti-fungal, and antibiotic activity [7-9]. However, the prospective of lignin is not clearly valued; because it is principally achieved as byproducts in pulp manufacture thus is mainly used as fuel. Fortunately, incorporating into polymeric materials will be a value-added relevance for lignin [10-12]. Lignins demonstrate a strong potential toward adsorption on organic substrates [13, 14]. In fact, extensive work has been conducted regarding the investigation of the properties of polymer/lignin/carbon filler composites [15, 16]. They are also employed in strengthening, electrostatic discharge, and sorption materials [17, 18]. Especially in rubber-based materials as compared to carbon black, lignin is less dense, non-conducting, and being lighter in color. For the preparation of light-colored rubber compounds it appears to be more agreeable [19]. The reinforcing consequence of lignin for polymer compounds widely depends on the particle size and strong interfacial bonding with matrix. The literature regarding the reinforcing effects of lignin on the polymer matrix composites have been reported [20]. Commonly, dry-milling and co-precipitation are mostly used for the preparation of lignin-filled rubber composites. Lignin simply milled as dry powder into the polymer displays almost no reinforcing effect. This is supposed to be a result of the lignin particles adhering together by intermolecular hydrogen bonding and thus not being dispersed into the polymer by milling. It is a unique property because of the ionization of the phenolic hydroxyl and carboxylic groups that lignin is soluble in aqueous alkali. Such solutions are compatible with the polymer latex in all proportions. To the best of our knowledge, the nanoscale lignin-CNT nanofiller reinforced in polyamide is novel. Therefore, we highlighted the fabrication of lignin-CNT nanofiller reinforced nylon 6 composites by simple technique. The effect of lignin coated carbon nanotube reinforcement on the thermal and mechanical properties of nylon 6 composite was studied. The effect of sonication time as well as filler content on the properties of the composites was studied.
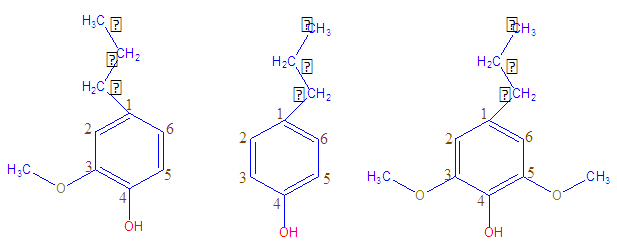 | Figure 1. Phenylpropanoid units of lignin |
2. Experimental
2.1. Materials
- Carbon nanotube, multi-walled (>98% carbon basis, O.D. × L 6-13 nm × 2.5-20 μm), lignin, alkali, and 1,4-dioxane anhydrous (99.8%) were purchased from Sigma. ε-Caprolactam, polyoxyethylene (POE) and N-acetylcaprolactam were purchased from Fisher Scientific.
2.2. Instrumentation
- The number average (Mn) and weight-average molecular weight (Mw) was measured using Viscotek TDAmax. Inherent viscosity (ηinh) was measured using an Ubbelohde viscometer using polymer solutions with a concentration of 0.5 g/dL. Differential scanning calorimetry (DSC) was performed by a METTLER TOLEDO DSC 822 differential scanning calorimeter at a rate of 10C/min under nitrogen atmosphere. Tensile testing was carried out using WP 310 universal material tester.
2.3. Purification of Carbon Nanotube
- 2 g of above carbon nanotube was added in 50 mL HCL and sonicated for 4 h at 30°C. Then the mixture was poured into large quantity of distilled water and filtered through vacuum filtration pump using polycarbonate filter paper. After the filtration, nanotube was washed with distilled water until the neutral pH was attained. The purified CNT was dried at 80°C for 6 h [21].
2.4. Lignin Coating of CNT (L-CNT)
- 0.1g CNT was dispersed in 50 mL of water with sonication of 2h. 1g lignin was dissolved in 50 mL dioxane containing 10% of H2O and few drops of HCl. The lignin solution was added dropwise to the CNT dispersion and sonicated for 6 h at 30°C. The mixture was then filtered and product was dried at 80°C for 6 h [22].
2.5. Preparation of Nylon-6
- A mixture of ε-caprolactam (100 g), polyoxyethylene (1g), and N-acetylcaprolactam (50 mL) was refluxed at 60°C for 1 h. The temperature was raised to 120°C and 1 g of NaH was added. The heating was continued for 1 h until the mixture became viscous [23].
2.6. Preparation of Nylon-6/L-CNT Composite
- Similar to the procedure mentioned in Section 2.5, a mixture of ε-caprolactam, polyoxyethylene, and N-acetylcaprolactam was prepared and refluxed at 60°C. Then at 120°C, NaH was added. After that the desired amount of modified carbon nanotube was added and the mixture was sonicated for preferred time (0.1-1 h) to obtain the composites [24].
3. Results and Discussion
3.1. Effect of Sonication Time and L-CNT Content on Viscosity and Molecular Weight
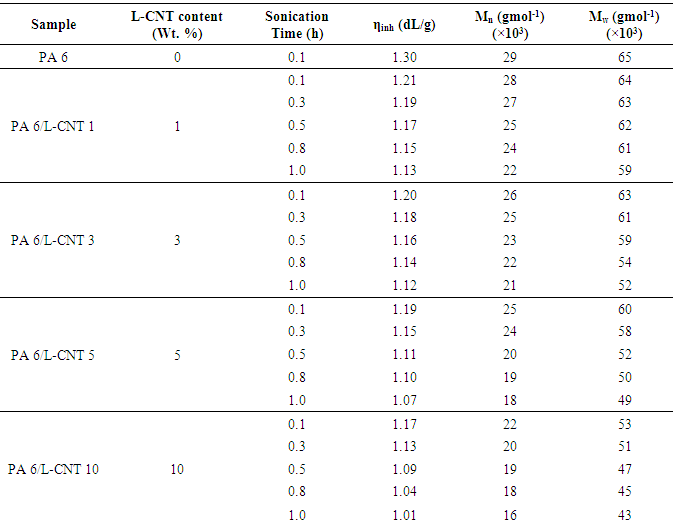
- Influence of sonication time as well as L-CNT content on the viscosity and molecular weight of the nanocomposite is given in Table 1. According to the viscosity measurement, L-CNT loading decreased the viscosity of the composites. The effect was more pronounced in the case of higher filler content as well as higher sonication time. In PA 6/L-CNT 10 composites, there was decrease of inherent viscosity from 1.17 to 1.01 dL/g with the increase in the sonication time from 0.1 to 1 h. On the other hand, PA 6/L-CNT 1 composite showed inherent viscosity decrease from 1.21 up to 1.13 dL/g with increase in sonication time. Therefore it was obvious that the filler addition as well as sonication time both were the factors affecting the molecular weight of the composites. The effect of lignin-CNT filler addition on the molecular weight of the polyamide was also studied using gel-permeation chromatography technique. To understand the effect of filler on the molecular weight of the synthesized polymer, dilute samples were prepared. The results supported the inherent viscosity study. The least weight average molecular weight decrease was observed in the case of PA 6/L-CNT 1 composite from 64 to 59 gmol-1 ×103 with increasing sonication time. Again 10 wt. % filler loading caused greater decrease in the molecular weight from 53 to 43 gmol-1 ×103. However, the values were considerably higher to represent a reasonable molecular weight. Similar decreasing trend with increasing loading was observed in the case of the number average molecular weight. Consequently, it was concluded that the addition of L-CNT has effected the polymerization of polyamide during in-situ process [23]. The mechanism behind seemed to be the hindrance of polymer chains growth due to the addition of rigid phase. L-CNT was supposed to cease the longer chain growth of the emergent polymer chain. Similarly increased sonication time disturbed the growth of the polymer chains via formation of smaller polymer segments.
|
3.2. Effect of Parameters on Glass Transition Temperature
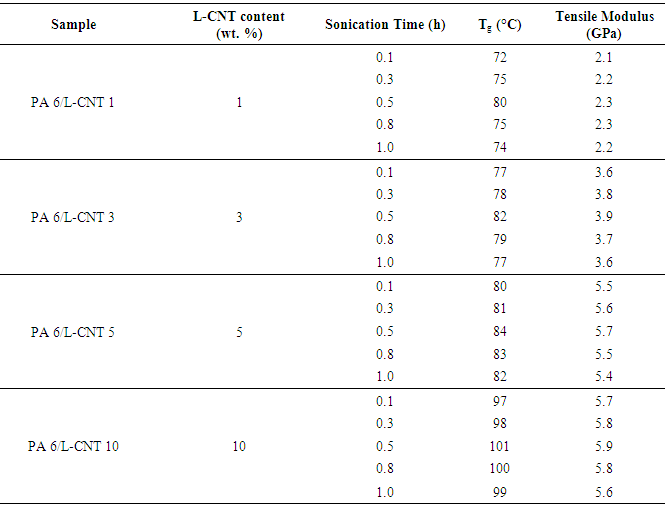
- Fig. 2 shows the DSC melting curves of PA 6/L-CNT composites obtained after 0.5 h sonication. The results showed that there was notable effect of L-CNT addition on the melting temperature of the composites (Table 2). All the materials prepared showed increase in glass transition temperature with rise in filler content as well as increase in sonication time upto 0.5 h. Above 0.5 h sonication, all the composites showed decrease in property. The obvious fact was the decomposition of polymer/CNT network upon increase in the sonication time in these composites. The results mean that 0.5 h was the optimum time required for the formation of these composites. The glass transition temperature for PA 6/L-CNT 1, PA 6/L-CNT 3, PA 6/L-CNT 5, and PA 6/L-CNT 10 was found as 80, 82, 84, and 101°C at 0.5 h sonication time. There was 21% increase in the Tg upon the addition of 10 wt. % filler compared with 1 wt. % L-CNT addition. The inclusion of nanofiller tends to endorse rigid structure to the polymer chains via matrix filler interaction [24, 25]. In other word, the amorphous form of the polymer was affected by the filler addition.
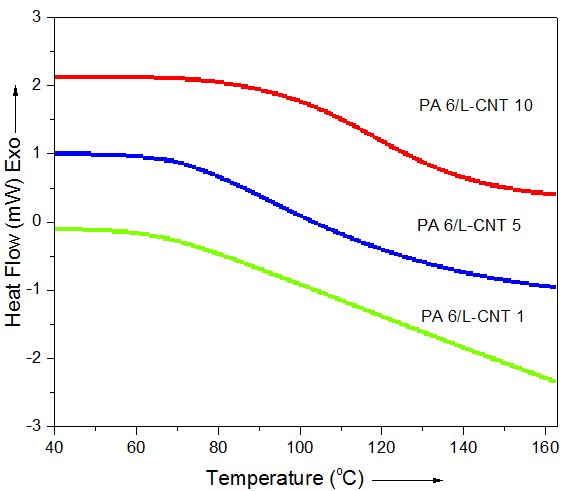 | Figure 2. DSC curves of PA 6/L-CNT composites after 0.5 h sonication |
|
3.3. Influence on Tensile Modulus
- Generally the addition of filler is known to increase the mechanical properties of the composites [26, 27]. In this attempt, the nanocomposite samples with various nanofiller addition (1-10 wt.%) were prepared by in-situ route (Table 2). Fig. 3 shows the tensile strength of PA 6/L-CNT nanocomposite at 0.5 h sonication. According to the results, the tensile modulus of the composites gradually increased with the filler addition. The tensile modulus of PA 6/L-CNT 1 nanocomposite was found as 2.3 GPa at 0.5 h. At longer sonication times, the modulus was found to decrease to 2.2 GPa. The tensile modulus was found to increase with filler loading. In PA 6/L-CNT 10 nanocomposite, the tensile modulus was increased to 5.9 GPa with 10 wt. % filler loading. Here again better results was obtained with 0.5 h sonication and longer sonication times decreased the property to 5.6 GPa. However there was 61% increase in the tensile modulus with 10 wt. % nanofiller addition compared with 1 wt. % L-CNT. The increase in mechanical properties with filler addition may be attributed to hydrogen bonding present between the functional carbon nanotube and polyamide.
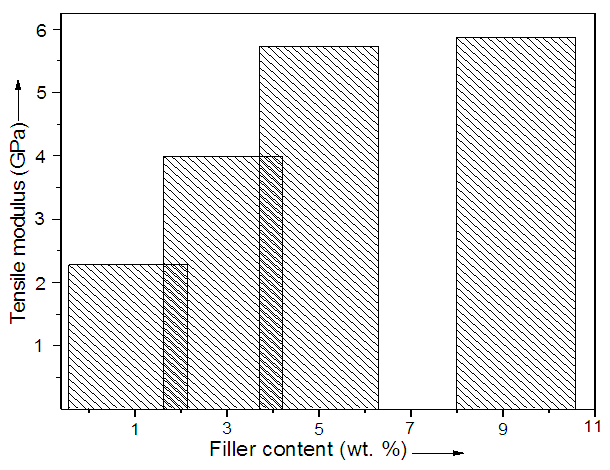 | Figure 3. Tensile modulus of PA 6/L-CNT nanocomposite after 0.5 h sonication |
4. Conclusions
- The lignin coated carbon nanotube was successfully used in the in-situ polymerization of nylon 6. The synthesis of in-situ polyamide/modified CNT is a perspective method from the technological point of view. In these composites, the lignin modified carbon nanotube was used as primary reinforcement. The introduction of L-CNT during in-situ polymerization of nylon 6 was found to be a very effective technique to form polyamide/L-CNT composites. Effect of sonication time as well as filler content was observed on the molecular weight, Tg and tensile modulus of the composites. The molecular weight of in-situ polymerized PA6 was decreased with the addition of L-CNT as well as increase in sonication time. Significant increase in the mechanical properties was observed up to the sonication time of 0.5 h. The tensile modulus of the composites was increased to 5.9 GPa in PA 6/L-CNT 10 nanocomposite. DSC studied indicated an increase in the glass transition temperature of PA 6/L-CNT due to increased chain rigidity indicating the presence of strong interaction between modified CNT and polyamide.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML