| [1] | N. Dkhireche, R. Abdelhadi, M. Ebn Touhami, H. Oudda, R. Touir, M. Sfaira, B. Hammouti, O. Senhaji, R. Taouil, Int. J. Electrochem. Sci., 7 (2012) 5314. |
| [2] | B. Mernari, H. El attari, M. Traisnel., F. Bentiss and M. Lagrenee, (1998), Corrosion, Vol. 40, p. 391. Ref brribri ss bentiss. |
| [3] | M. Lagrenee, M. Traisnel, B. Mernari, F. Benstiss, H. El Attari, Journal of Applied Electrochemistry 29 1073-1078, (1999). |
| [4] | M. El Achouri, S. Kertit, M. Salem, E.M. Essassi, M. Jellal, (1998), Bull. Electrochem., Vol. 14 No.12, pp.462–8.23. |
| [5] | B. El Mehdi, B. Mernari, M. Traisne, F. Bentiss, M. Lagrenée, Mater. Chem. Phys., 77 (2003) 489–496. |
| [6] | F. Bentiss, C. Jama, B. Mernari, H. El Attari, L. El Kadi, M. Lebrini, M. Traisnel, M. Lagrenée, Corros. Sci., 51 (2009) 1628– 1635. 25. |
| [7] | K. Adardour, O. Kassou, R. Touir, M. Ebn Touhami, H. ElKafsaoui, H. Benzeid, E. Essassi, M. Sfaira J. Mater. Envir. Sci., 1 (2010) 129. |
| [8] | Y. Elkacimi, M. Achnin, Y. Aouine, M. Ebn Touhami, A. Alami, R. Touir, M. Sfaira, D. Chebabe, A. Elachqar, B. Hammouti, Portugaliae Electrochem. Acta 30, 53 (2012) |
| [9] | K. Adardour • R. Touir • M. Elbakri • Y. Ramli • M. Ebn Touhami • H. El Kafsaoui • C. Kalonji Mubengayi • E. M. Essassi, Res Chem Intermed DOI10.1007/s11164-012-0934-x,. |
| [10] | H. El Attari, A. El Bribri, L. Mhaidra, F. Bentiss and M. Siniti, American Journal of Engineering Research, Volume-4, Issue-3, pp-44-51. |
| [11] | A.A. Abdel Fattach, K.M. Atia, F.S. Ahmed, M.I. Roushdy, Corrosion Prevention and Control 33 (3) (1986) 67. |
| [12] | M. Elachouri, M.S. Hajji, M. Salem, S. Kertit, E.M. Essassi, Corrosion Science 37 (1995) 381. |
| [13] | M. Elachouri, M.S. Hajji, M. Salem, S. Kertit, J. Aride, R. Coudert, E. Essassi, Corrosion 52 (1996)103. |
| [14] | I.L. Rozenfeld, Corrosion Inhibitors, McGraw-Hill, New York, 1981. |
| [15] | B.A. Abd-EI-Nabey, E. Khamis, M.Sh. Ramadan, A. EI-Gindy, Corrosion 52 (1996) 671. |
| [16] | A.K. Maayta, M.B. Bitar, M.M. Al-Abdallah, Brit. Corros. J. 36 (2001) 133. |
| [17] | R. Fuchs-Godec, V. Dolecek, Colloids Surf. A 244 (2004) 73. |
| [18] | R.F.V. Villamil, P. Corio, J.C. Rubim, M.L. Silvia Agostinho, J. Electroanal. Chem. 472 (1999) 112. |
| [19] | S.S. Abd El Rehim, H.H. Hassaan, M.A. Amin, Mater. Chem. Phys. 78 (2002) 337. |
| [20] | A.K. Maayta, N.A.F. Al-Rawashdeh, Corros. Sci. 46 (2004) 1129. |
| [21] | R. Guo, T. Liu, X. Wei, Colloids Surf. A 209 (2002) 37. |
| [22] | V. Branzoi, F. Golgovici, F. Branzoi, Mater. Chem. Phys. 78 (2002) 122. |
| [23] | M. Elachouri, M.S. Hajji, M. Salem, S. Kertit, J. Aride, R. Coudert, E. Essassi, Corrosion 52 (1996) 103. |
| [24] | A.S. Algaber, E.M. El-Nemma, M.M. Saleh, Mater. Chem. Phys. 86 (2004) 26. |
| [25] | M.M. Osman, M.N. Shalaby, Anti-Corros. Methods Mater. 44 (1997) 318. |
| [26] | M.M. Osman, A.M.A. Omar, A.M. Sabagh, Materials Chemistry and Physics 50 (1997) 271. |
| [27] | F. Hanna, G.M. Sherbini, Y. Brakat, British Corrosion Journal 24 (1989) 269. |
| [28] | M.M. Osman, M.N. Shalaby, Materials Chemistry and Physics 77 (2002) 261. |
| [29] | F.N. Speller, Corrosion Causes and Prevention, McGraw-Hill, New York, 1951. |
| [30] | M.M. Osman, M.N. Shalaby, Anti-Corrosion Methods and Materials 44 (5) (1997) 318. |
| [31] | D. Gobi, N. Bhuvaneswaran, S. Rajeswarai, K. Ramadas, Anti-Corrosion Methods and Materials 47 (6) (2000) 332. |
| [32] | A. El bribri, M. Tabyaoui, H. El Attari, K. Boumhara, M. Siniti, B. Tabyaoui J. Mater. Environ. Sci. 2 (2) (2011) 156-165. |
| [33] | M. Elachouri, M.S. Hajji, M. Salem, S. Kertit, J. Aride, R. Coudert, E. Essassi, Corrosion 52 (1996) 103. |
| [34] | M. Bouklah, N. Benchat, B. Hammouti, A. Aouniti, S. Kertit, Mater. Lett. 60 (2006) 1903. |
| [35] | M.M. Osman, A.M.A. Omar, A.M. Sabagh, Materials Chemistry and Physics 50 (1997) 271. |
| [36] | A. El bribri, H. El attari, M SINIT and M. Tabyaoui Mor. J. Chem. 3 N°2 (2015) 286 297. |
| [37] | H. Elmsellem, H. Bendaha, A. Aouniti, A. Chetouani, M. Mimouni, A. Bouyanzer, Mor. J. Chem. 2 N°1 (2014) 1-9. |
| [38] | F. El-Hajjaji, R.A. Belkhmima, B. Zerga, M. Sfaira, M. Taleb, M. Ebn Touhami, B. Hammouti, J. Mater. Environ. Sci. 5 (1) (2014) 263-270. |
| [39] | Chris O. Akalezi, Conrad K. Enenebaku, Emeka E. Oguzie, J. Mater. Environ. Sci. 4 (2) (2013) 217-226. |
| [40] | L.B. Tang, G.N. Mu, G.H. Liu, Corros. Sci. 45 (2003) 2251. |
| [41] | E. Khamis, Corrosion 46 (1990) 476. |
| [42] | A. El Awady, A. Abd El Naby, S. Aziz, J. Electrochem. Soc. 139 (1992) 2149. |
| [43] | E. Cano, J.L. Polo, A. La Iglesia, J.M. Bastidas, Adsorption 10 (2004) 219. |
| [44] | G. Moretti, F. Guidi, G. Grion, Corros. Sci. 46 (2004) 387. |
| [45] | D. Do, Adsorption Analysis: Equilibria and Kinetics, Imperial College Press, 1998, pp. 10–60. |
| [46] | S.A. Ali, A.M. El-Shareef, R.F. Al-Ghamdi, M.T. Saeed, Corros. Sci. 47, 2659 (2005). |
| [47] | L. Tang, X. Li, Y, Si, G. Mu and G. Liu, Mater. Chem. and Phys., 95 (2006) 29. |
| [48] | E. E. Ebenso. Mater. Chem. and Phys., 79 (2003) 58. |
| [49] | G. E., Badr, Corros. Sci., 51 (11), (2009) 2539. |
| [50] | T.P. Zhao, G.N. Mu, Corros. Sci. 41 (1999) 1937. |
| [51] | L.B. Tang, G.N. Mu, G.H. Liu, Corros. Sci. 45 (2003) 2251. |
| [52] | Badiea A. Mohammed, Kikkeri N. Mohana, Monatsh Chem/Chemical Monthly 140 (2009) 1–8. |
| [53] | Chandra Bhan Verma, M.A. Quraishi1, E.E. Ebenso Int. J. Electrochem. Sci., 9 (2014) 5507 - 5519, |
| [54] | I Bockris, J.O.M., Reddy, A.K.N. Modern Electrochemistry, vol. 2, Plenum Press, New York, 1977, p.1267. |
| [55] | I. Ahamad. R. Prasad and M.A. Quraishi Corros. Sci. 52 (2010) 1472–1481. |
| [56] | Sami Ben Aoun, Der Pharma Chemica, 5(3) (2013), 294-304. |
| [57] | Deng X. Li, H. Fu, G. Mu, Corros. Sci. 50 (2008)2635– 2645. |
| [58] | O. Riggs, I.R. Hurd, M. Ray, Corrosion 23, (1967) 252. |
| [59] | Szauer T., Brandt A., Electrochim. Acta 26 (1981) 1209. |
| [60] | S.N. Banerjee, S. Misra, Corrosion 45, 780 (1989). |
| [61] | Q.H. Cai, Y.K. Shan, B. Lu, X.H. Yuan, Corrosion 49, 486 (1993). |
| [62] | A. Zarrouk, A. Chelfi, A. Dafali, B. Hammouti, S.S. Al-Deyab, I. Warad, N. Benchat, M. Zertoubi, Int. J. Electrochem. Sci. 5, 696 (2010). |
| [63] | J. Marsh, Advanced organic chemistry, 3rd edn. (Wiley Eastern, New Delhi, 1988). |
| [64] | J.O.M Bockris, A.K.N, Reddy, Modern Electrochemistry, vol. 2, Plenum Press, New York, 1977, p.1267. |

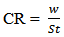
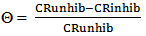
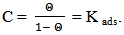
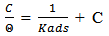
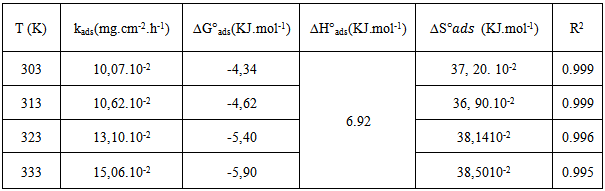
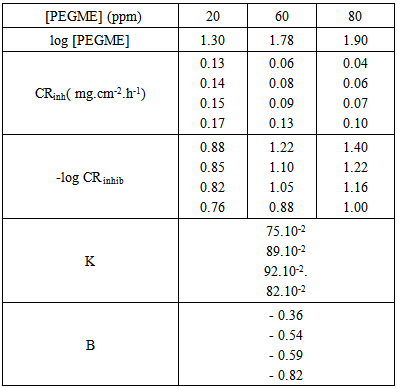
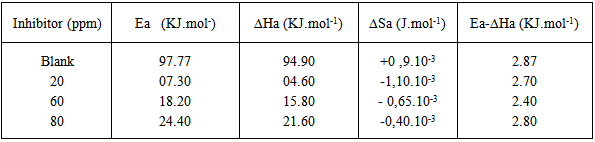
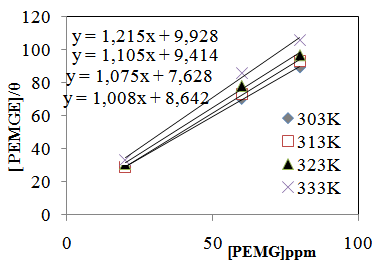
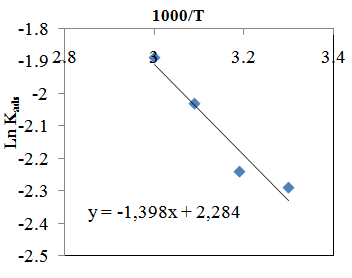
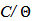 vs C with slopes very close to 1. The strong correlation (R2>0.999) suggested that the adsorption of the inhibitor molecules in 1N HCl on the metal surface obeyed to the Langmuir’s adsorption isotherm (Fig.5) [40]. As the adsorption isotherm in 1 N HCl is of Langmuir-type with slope of almost unity, monolayer of the inhibitor species must have been attached to mild steel surface without lateral interaction between the adsorbed species. The values of Kads were calculated from the intercepts of the straight lines on the C/θ – axis. The Kads was related to the standard free energy of adsorption, ΔG° ads according to the following equation [41]:
vs C with slopes very close to 1. The strong correlation (R2>0.999) suggested that the adsorption of the inhibitor molecules in 1N HCl on the metal surface obeyed to the Langmuir’s adsorption isotherm (Fig.5) [40]. As the adsorption isotherm in 1 N HCl is of Langmuir-type with slope of almost unity, monolayer of the inhibitor species must have been attached to mild steel surface without lateral interaction between the adsorbed species. The values of Kads were calculated from the intercepts of the straight lines on the C/θ – axis. The Kads was related to the standard free energy of adsorption, ΔG° ads according to the following equation [41]: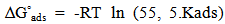
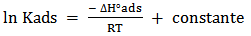
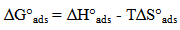
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML