-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2015; 5(3): 64-76
doi:10.5923/j.ijmc.20150503.03
Kinetics and Thermodynamic Study of Inhibition Potentials by Ethoxyethane Extracts of Cochlospermum tinctorium for the Oxoacid Corrosion of Mild Steel
Kazaure Z. Sulay1, Agbogo U. Victor2, Bognet Obed3, Adeyemi O. Olufemi4
1Brigadier General, PhD Pure and Applied Chemistry, Nigerian Defence Academy, Kaduna, Nigeria
2Masters Degree in Materials Science and Explosives, Chemistry Department, Nigerian Defence Academy, Kaduna, Nigeria
3Masters Degree in Inorganic Chemistry, Chemistry Department, Nigerian Defence Academy, Kaduna, Nigeria
4Associate Professor of Physical Chemistry, Olabisi Onabanjo University, Ogun State, Nigeria
Correspondence to: Agbogo U. Victor, Masters Degree in Materials Science and Explosives, Chemistry Department, Nigerian Defence Academy, Kaduna, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The corrosion inhibition efficiency of a Cochlospermum tinctorium (CTE) on mild steel in 0.5M H2SO4 solution has been investigated using weight loss measurements, thermometric measurement and scanning electron microscopy studies. The weight loss measurement indicates increase corrosion inhibition efficiencies that reach 97%. The weight loss and thermometric data established that the inhibition efficiency on mild steel increases with increase in the concentration of inhibitor, CTE. The adsorption of CTE obeys Langmuir adsorption isotherm. Thermodynamic parameters 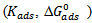 were calculated using the adsorption isotherm. Activation parameters of the corrosion process
were calculated using the adsorption isotherm. Activation parameters of the corrosion process 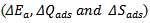 were also calculated from the corrosion rates obtained from temperature studies. The adsorption isotherm indicates that the adsorption of CTE inhibitor on the surface of mild steel is physisorption and the thermodynamic values obtained indicates spontaneous and exothermic corrosion processes.Furthermore, this thesis includes the thermometric study for the mild steel corrosion in 0.5M H2SO4 acid solution in absence and presence of different CTE concentrations as inhibitor. Thermometric measurements data show that adding CTE to 0.5M H2SO4 and acid solution leads to decrease in the reaction number RN and the percentage reduction in RN in the presence of CTE increases with increasing CTE concentrations, therefore the Inhibition efficiency increases with increasing CTE concentration and it reaches to the highest value 90% by using 500 mgL-1 of CTE.Surface morphology of the mild steel specimens in the presence and absence of the inhibitor was evaluated by SEM analysis. SEM analysis revealed that the addition of inhibitor retarded the corrosion processes, where the grain boundary attacks were completely hindered by the adsorbed inhibitor molecules. The micrograph in the presence of CTE showed a near smoother surface with pit morphology lower than in the absence of plant extract.
were also calculated from the corrosion rates obtained from temperature studies. The adsorption isotherm indicates that the adsorption of CTE inhibitor on the surface of mild steel is physisorption and the thermodynamic values obtained indicates spontaneous and exothermic corrosion processes.Furthermore, this thesis includes the thermometric study for the mild steel corrosion in 0.5M H2SO4 acid solution in absence and presence of different CTE concentrations as inhibitor. Thermometric measurements data show that adding CTE to 0.5M H2SO4 and acid solution leads to decrease in the reaction number RN and the percentage reduction in RN in the presence of CTE increases with increasing CTE concentrations, therefore the Inhibition efficiency increases with increasing CTE concentration and it reaches to the highest value 90% by using 500 mgL-1 of CTE.Surface morphology of the mild steel specimens in the presence and absence of the inhibitor was evaluated by SEM analysis. SEM analysis revealed that the addition of inhibitor retarded the corrosion processes, where the grain boundary attacks were completely hindered by the adsorbed inhibitor molecules. The micrograph in the presence of CTE showed a near smoother surface with pit morphology lower than in the absence of plant extract.
Keywords: Corrosion, Mild Steel, Inhibition, Cochlospermum tinctorium, Surface morphology
Cite this paper: Kazaure Z. Sulay, Agbogo U. Victor, Bognet Obed, Adeyemi O. Olufemi, Kinetics and Thermodynamic Study of Inhibition Potentials by Ethoxyethane Extracts of Cochlospermum tinctorium for the Oxoacid Corrosion of Mild Steel, International Journal of Materials and Chemistry, Vol. 5 No. 3, 2015, pp. 64-76. doi: 10.5923/j.ijmc.20150503.03.
Article Outline
1. Introduction
- Corrosion is the deterioration of materials by chemical interaction with their environment. It is the destruction or deterioration of a material because of its reaction with its environment [11]. The term corrosion is sometimes also applied to the degradation of plastics, concrete, and wood, but generally refers to metals. The most widely used metal is iron (usually as steel). Corrosion can cause disastrous damage to metal and alloy structures causing economic consequences in terms of repair, replacement, product losses, safety and environmental pollution. Due to these harmful effects, corrosion is an undesirable phenomenon that ought to be prevented [20]. Corrosion control of metals is technically, economically, environmentally and aesthetically important. The best option is to use inhibitors for protecting metals and alloys against corrosion. As inorganic corrosion inhibitors are toxic in nature, so green inhibitors which are biodegradable without any heavy metals and other toxic compounds are promoted. Also plant products are inexpensive, renewable, and readily available. Tannins, organic amino acids, alkaloids and organic dyes of plant origin have good corrosion-inhibiting abilities. Plant extracts contain many organic compounds, having polar atoms such as O, P, S and N. These are adsorbed on the metal surface by these polar atoms and protective films are formed and various adsorption isotherms are obeyed [26].This study is therefore focused at evaluating the anti-corrosive activities of its diethyl ether extract in acidic medium, H2SO4. This is because there is presence of different chemical constituents in cochlospermum tinctorium from phytochemical screening. Organic amino acids, alkaloids, and organic dyes of plant origin have good corrosion-inhibiting abilities. Plant extracts contain many organic compounds, having polar atoms such as O, P, S, and N. These are adsorbed on the metal surface by these polar atoms, and protective films are formed and various adsorption isotherms are obeyed [21].
2. Research Experimental
2.1. Collection and Extraction of the Plant Sample
- Samples of Cochlespermum tinctorium were collected from Abocho, Dekina Local Government Kogi State, identified in University of Calabar Herbarium with voucher number 029 and family name Cochlospermacae and it was sun dried and grounded to powdered form, 300 g of the pulverized sample was weighed and soaked in diethyl ether for 48 hours. After 48 hours, the filtrate was distilled in a water bath that was maintained at 35°C in order to leave the extract free of diethyl ether.The diethyl ether free extract was used in the preparation of the test solutions by dissolving 0.1g, 0.2g, 0.3g, 0.4g, 0.5g of the extract in 1L of 0.5M H2SO4 [15] and then used to test for corrosion inhibition properties. This is because the inspection of cochlospermum tinctorium by Ahmad 2011 and Etuk et al., 2009 was believed to contain phytochemicals that show the presence of heterotatoms which could serve as a source of bonding to metal surface to reduce corrosion processes in metals[17, 18].
2.2. Materials Preparation
- Materials used were collected from Naval dock Yard Apapa, Lagos. The metals were subjected to elemental analysis. The sheet were mechanically press cut into different coupons, each of dimension 4.0 x 2.0 x 2cm2 rectangular specimens and polished with emery papers (silicon carbide paper, 100- 1200) grades.These polished coupons were subjected to water test in agreement with ASTM 2000 to make sure the metal surfaces were free of pits. Each coupon were degreased by washing with and dipped in acetone and allowed to dry and was preserved in a desiccators. All reagents that were used for the study were of analar grades and double distilled water. The prepared coupons were now subjected to corrosion testing using;1. Gravimetric technique2. Thermometric technique3. Surface morphological studiesThe concentrations of the inhibitors were within the range, 1 to 5mg/l. Each of these concentrations were used to prepare different test solutions by dissolving them in 0.5 H2SO4 for use in the various analysis.
2.3. Gravimetric Method of Analysis
- The research studies were carried out by experiment, a weighed metal (mild steel) coupon was completely immersed in 250 ml of the test solution in an open beaker. The beaker was cover with aluminium foil and then inserted into a water bath that was maintained at 303 K. The corrosion product was removed after some hours intervals by washing each coupon (withdrawn from the text solution) in a solution containing 50% NaOH (sodium hydroxide) and 100gL-1 of zinc dust [13]. The washed coupon were rinsed in acetone and dried in the air before re-weighing. The experiments were repeated at 330k [16]. In each case the difference in weight for a period of various hours were taken as the total weight loss from the average weight loss results (average of three replicator, the corrosion rate of mild steel and the degree of surface coverage were calculated using the Equation (3), (4) (5) respectively [19]
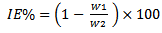 | (3) |
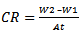 | (4) |
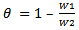 | (5) |
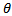 is the degree of surface coverage of the inhibitor.
is the degree of surface coverage of the inhibitor.2.4. Thermometric Method
- Measurement of temperature was carried out according to the method described by Ebenso and Eddy 2008. From the rise in temperature of the system per minute the reaction number (RN) and the inhibition efficiency were calculated using equation 6 and 7 [19]
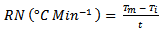 | (6) |
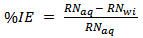 | (7) |
2.5. Surface Morphological Studies
- Surface analysis was performed using scanning electron microscope. SEM images were obtained from MS surface after the immersion in 0.5 H2SO4 in the absence and presence of cochlospermum tinctorium for two hours at 30°C.
2.5.1. Micro Structural Examination
- The microstructure of the mild steel coupons were examined before and after the corrosion experiment to assess the effect of corrosion on the microstructure and the effect of the addition of the extract as an inhibitor. The process series of preparatory stages carried out before the actual microscopic examination are sectioning, mounting, rough grinding, smooth grinding, polishing and etching.Micro structural examination was done by using the metallurgical microscope. The etched specimen was placed on the microscope and the microstructure of the various coupons was obtained accordingly.
3. Results and Discussion
3.1. Weight Loss Measurement
- The loss in weight of mild steel in the presence and absence of cochlospermum tinctorium at different temperatures and immersion time intervals was determined, Table 1. From the Table, it was evident that there was a metal dissolution in the presence and absence of plant extract. It is pertinent to note however that the weight loss was higher in the absence of the plant extract than its presence.
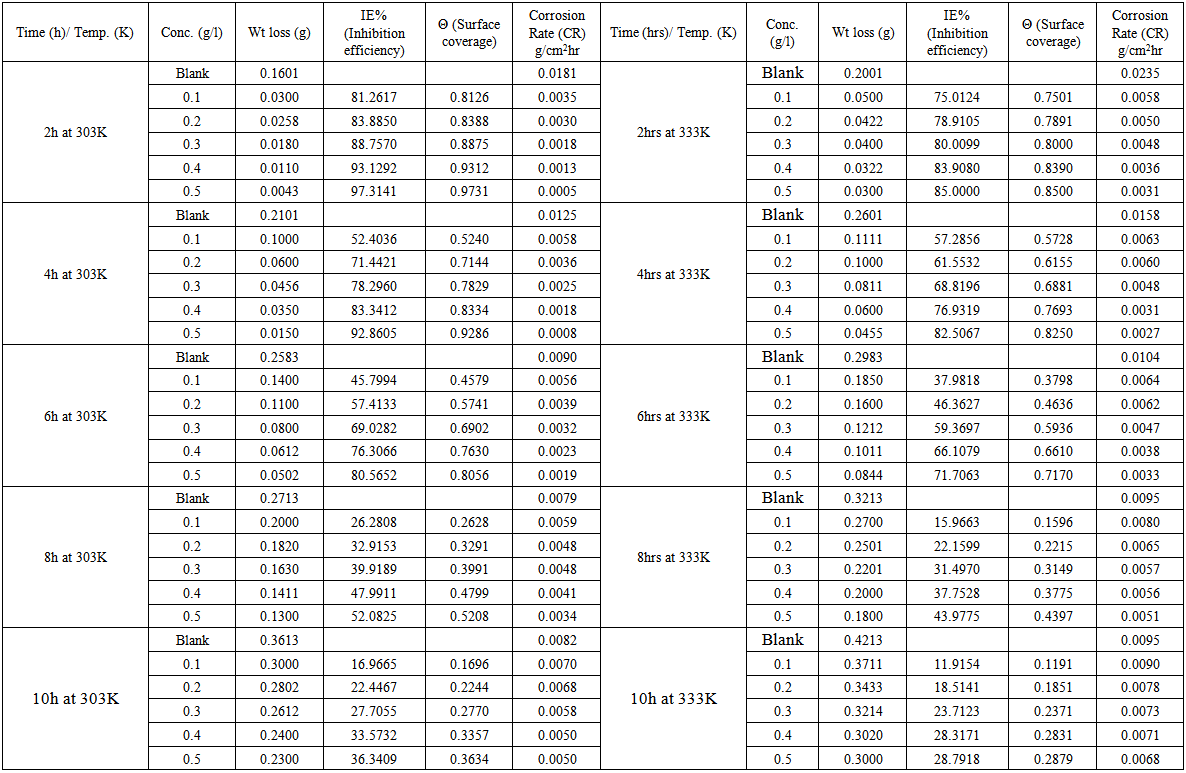 | Table 1. Weight loss, inhibition efficiencies, surface coverage and corrosion rate of mild steel in 0.5M H2SO4 in the presence and absence of extract of Cochlospermum tinctorium at varied temperature |
3.2. Inhibition Efficiency and Degree of Surface Coverage
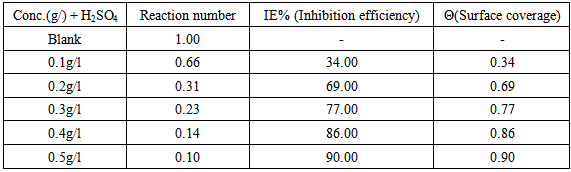
- Inhibition efficiency and surface coverage of Cochlospermum tinctorium in 0.5M H2SO4 at 303 K-333 K was determined at several intervals in the presence of various concentrations of CTE inhibitor. These results are shown in Table 1. At the inhibitor concentration of 0.5g/dm-3, the maximum IE% was 97.3141% for 0.5M H2SO4 all which shows that CTE act as a good corrosion inhibitor for mild steel in acid corrodents. This result suggests that the increase in efficiencies with increase in inhibitors concentration was due to increase of the number of molecules adsorbed onto mild steel surface and reduces the surface area that is available for the direct acid attack on the metal surface, this agree with the work of Afia et. al., 2013 [25]. Adeyemi and Singh 1987 observed that some inhibitors are effective at short intervals and reduces inhibitive properties with time because of autocatalysis. The same observation was made in the present case. CTE exhibited higher inhibition at 97.3141% in immersion. The higher corrosion inhibition efficiency is due to the higher surface coverage. This is, so we assumed that the inhibitor molecules formed a protective blanket on the metal/solution interface [24].
3.3. Effect of Temperature
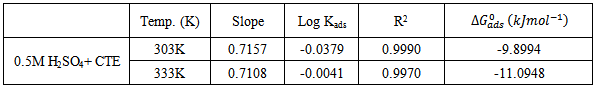
- The effect of temperature on the inhibitive efficiency of CTE inhibitor in 0.5 M H2SO4 at temperature in the range (303 – 333) K was studied. Temperature has a great effect on the corrosion phenomenon. Generally the corrosion rate increases with rising of the temperature. For this purpose, the temperature was varied in the range of 303 to 333 K, in the absence and presence of CTE. The corresponding data are shown in Fig 1.; it is clear that the increase of corrosion rate is more pronounced with the rising temperature for blank solution, this is to say that the higher the corrosion inhibition efficiency the lower the corrosion rate.For each inhibitor that concentration was selected which showed higher efficiency at 303 K. The results which given in Table 2. show that in plain as well as in acid containing CTE the corrosion rate increases and as a result the inhibition efficiency decreases with a rise in temperature as shown in Fig. 1. This may be attributed to desorption of the inhibitors molecules at higher temperatures, thus exposing the metal surface to further attack.
|
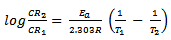 | (8) |
|
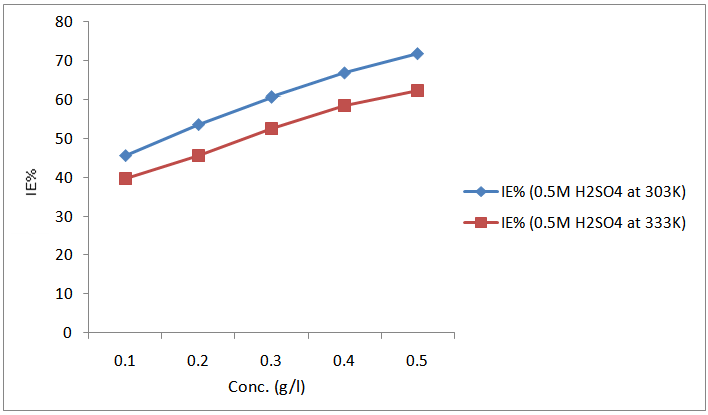 | Figure 1. Variation of inhibition efficiency for mild steel with different concentration of CTE inhibitor in 0.5 M H2SO4 at 303 K and 333 K |
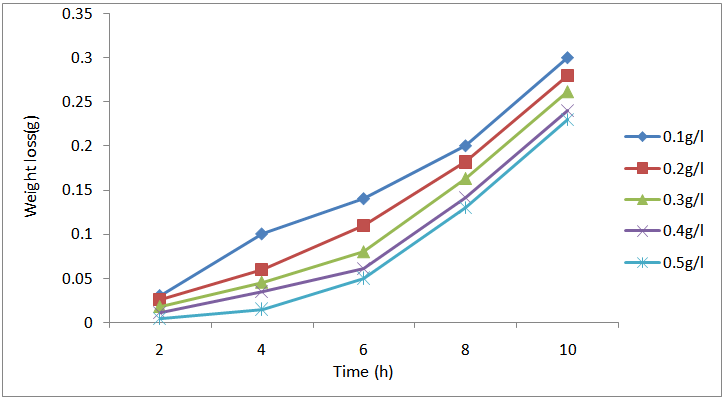 | Figure 2. Variation of weight loss with time for the corrosion of mild steel in 0.5M H2SO4 containing the various concentrations of CTE at 303 K |
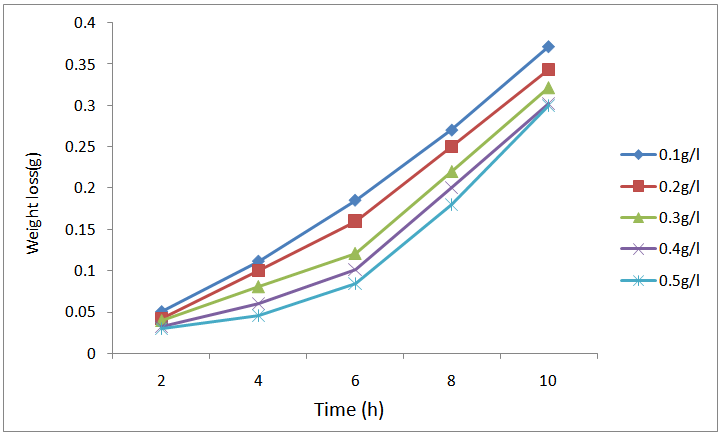 | Figure 3. Variation of weight loss with time for the corrosion of mild steel in 0.5M H2SO4 containing the various concentrations of CTE at 333 K |
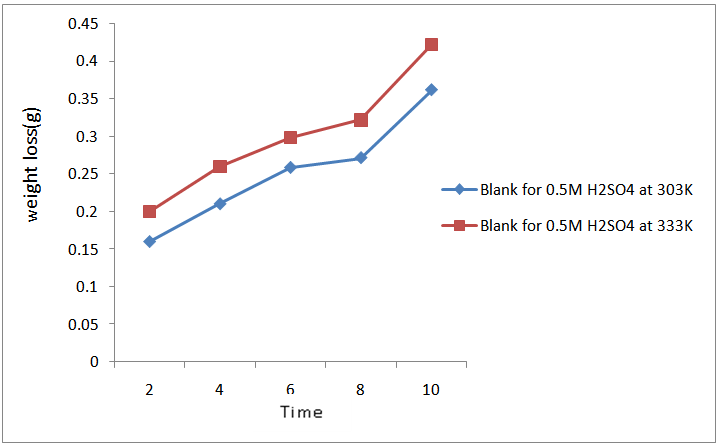 | Figure 4. variation of weight loss with time for the corrosion of mild steel in 0.5M H2SO4 in the absence of CTE inhibitor at 303K and 333K |
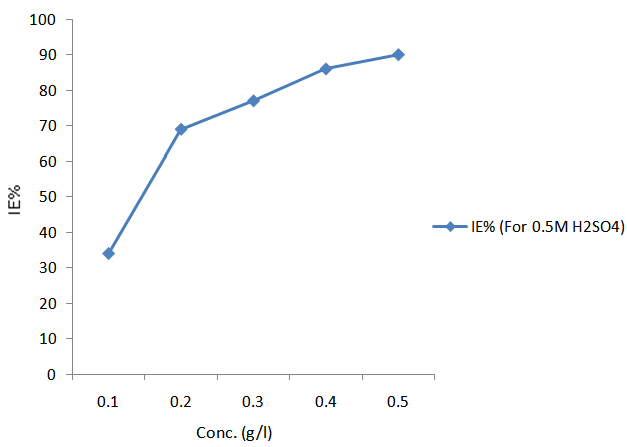 | Figure 5. Variation of inhibition efficiency with concentration for the corrosion of mild steel in 0.5M H2SO4 in the presence of CTE inhibitor for thermometric measurement |
3.4. Adsorption Isotherm
- The adsorption isotherm can be determined by assuming that inhibition effect is due mainly to the adsorption at metal solution interface. Basic information on the adsorption of inhibitors on the metal surface can be provided by adsorption isotherm. In order to obtain the isotherm, the fractional surface coverage values θ as a function of inhibitor concentration must be obtained. The values of θ can be easily determined from the weight loss and electrochemical measurements by the ratio %IE/100, where %IE is inhibition efficiency obtained by weight loss and thermometric measurement. So it is necessary to determine empirically which isotherm fits best to the adsorption of inhibitors on the mild steel surface. Several adsorption isotherms (viz., Langmuir, Freundlich, Temkin, Flory-Huggins, Bockris-Swinkel, El Awardy et. al., and Frumkin) were tested and the Langmuir adsorption isotherm was found to provide the best description of the adsorption behaviour of these inhibitors. The Langmuir isotherm is given by equation 9 [29]:
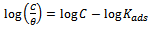 | (9) |
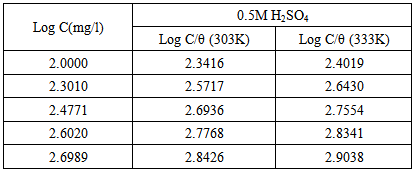
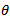 is the surface coverage. Plot log
is the surface coverage. Plot log 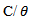 versus log C in fig 6. to fig 3.23 yields a straight line with slope and regression coefficient, R2, almost equal to unity. This suggests that CTE inhibitor in present study obeyed the Langmuir isotherm and there is negligible interaction between the adsorbed molecules. Free energy of adsorption was calculated using the relation in equation 10 [30].
versus log C in fig 6. to fig 3.23 yields a straight line with slope and regression coefficient, R2, almost equal to unity. This suggests that CTE inhibitor in present study obeyed the Langmuir isotherm and there is negligible interaction between the adsorbed molecules. Free energy of adsorption was calculated using the relation in equation 10 [30].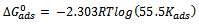 | (10) |
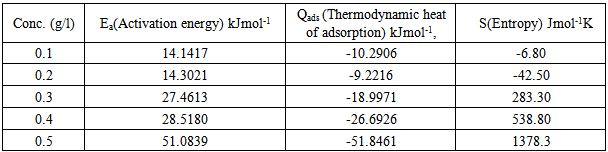
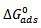 are also presented in table 4. from the results obtained, the free energies ranges from ranged from -9.8994 kJmol-1 to -11.0948 kJmol-1 for 0.5M H2SO4 and are negatively less than the threshold value of -40kJmol-1 required for the mechanism of chemical adsorption. Therefore, the adsorption of the CTE inhibitor on mild steel surface in sulphuric is spontaneous and favours the mechanism of adsorption. Generally, values of
are also presented in table 4. from the results obtained, the free energies ranges from ranged from -9.8994 kJmol-1 to -11.0948 kJmol-1 for 0.5M H2SO4 and are negatively less than the threshold value of -40kJmol-1 required for the mechanism of chemical adsorption. Therefore, the adsorption of the CTE inhibitor on mild steel surface in sulphuric is spontaneous and favours the mechanism of adsorption. Generally, values of 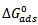 around -20kJ/mol are consistent with the electrostatic interaction between the charged molecules and the charged metal (physical adsorption) whereas those more negative than -40kJ/mol involves charge sharing or transfer from the inhibitors molecule to the metal surface leading to the formation of a donor-acceptor bond (chemical adsorption). For the natural specie investigated in this study,
around -20kJ/mol are consistent with the electrostatic interaction between the charged molecules and the charged metal (physical adsorption) whereas those more negative than -40kJ/mol involves charge sharing or transfer from the inhibitors molecule to the metal surface leading to the formation of a donor-acceptor bond (chemical adsorption). For the natural specie investigated in this study, 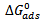 values, indicate that the adsorption of the inhibitors is physical. This is in good agreement with the value obtained by other workers [16, 19]. They observed in physical adsorption that the values of
values, indicate that the adsorption of the inhibitors is physical. This is in good agreement with the value obtained by other workers [16, 19]. They observed in physical adsorption that the values of 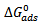 negative. Thus the process is a spontaneous one.
negative. Thus the process is a spontaneous one.
|
 | Figure 6. |
3.5. Thermodynamics/Adsorption Study
- The heat of adsorption of the inhibitors was calculated using equation 11,
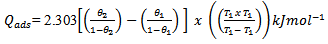 | (11) |
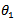 and
and 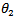 are the degrees of surface coverage of the inhibitor at the temperatures T1 (303 K) and T2 (333 K). Calculated values of Qads recorded in Table 3, are negative indicating that the adsorption of the studied amino acids on mild steel surface is exothermic [Sharma and Sharma1999]. Since the reactions were carried out at constant pressure, the value of
are the degrees of surface coverage of the inhibitor at the temperatures T1 (303 K) and T2 (333 K). Calculated values of Qads recorded in Table 3, are negative indicating that the adsorption of the studied amino acids on mild steel surface is exothermic [Sharma and Sharma1999]. Since the reactions were carried out at constant pressure, the value of 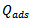 will be approximate of 𝝙Hads [Ulicks 1960]. The value of entropy, Sads will be determined using equation 12.
will be approximate of 𝝙Hads [Ulicks 1960]. The value of entropy, Sads will be determined using equation 12.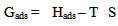 | (12) |
3.6. Thermometric Measurements
- The dissolution reaction of mild steel in 0.5M H2SO4 in the absence and presence of CTE inhibitor was also studied using thermometric method. This technique has proved to be of considerable value and help in studying corrosion behaviour of a number of metals and alloys in various corroding environments. The technique is also useful in evaluating the inhibitor efficiency of a number of surface-active agents [31]. Table 5 shows the result for the dissolution of mild steel in 0.5M acid corrodents in the absence and presence of different concentration of CTE.
|
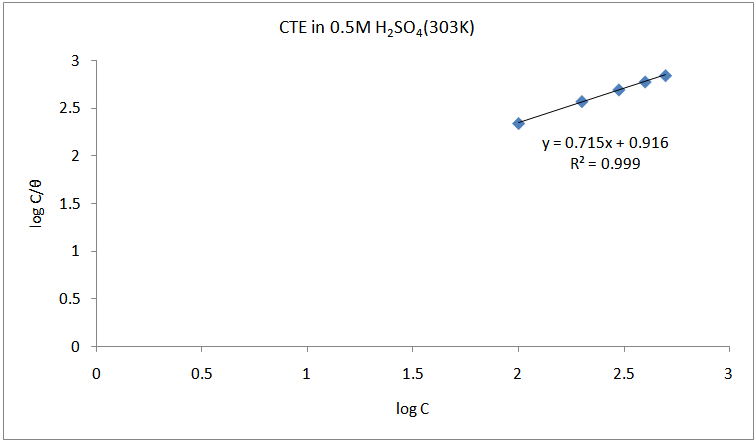 | Figure 7. Langmuir isotherm for the adsorption of CTE inhibitor on the surface of mild steel |
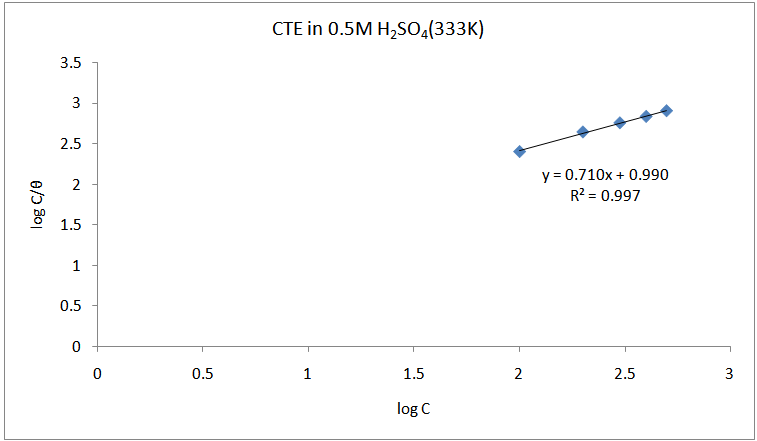 | Figure 8. Langmuir isotherm for the adsorption of CTE inhibitor on the surface of mild steel |
3.7. Metallographic Examination of Micrographs
- The surface morphology of the metal was evaluated by scanning electron microscopy (SEM) to show the micrographs of the freshly polished mild steel surface, corrosion specimens with and without CTE inhibitor in 0.5M H2SO4.The metallographic structural micrographs before and after immersion in plain acids and in presence of 0.5g/l plant extracts are shown as Figures 9 a, b, c, respectively. Fig. 9a shows plain metal surface before immersion. The surface shows a more uniform distribution of ferrite and pearlite structure in the grain structures with distinct grain boundary pits at a magnification of x500.From Fig. 9b the micrograph in 0.5M H2SO4 without inhibitor is shown, it is evident that there is a form of grain boundary attack by the acids.
 | Figure 9a. Micrograph for Freshly Polished MS before immersion |
 | Figure 9b. Micrograph for MS in 0.5M H2SO4 without CTE |
 | Figure 9c. Micrograph for MS in 0.5M H2SO4 with CTE |
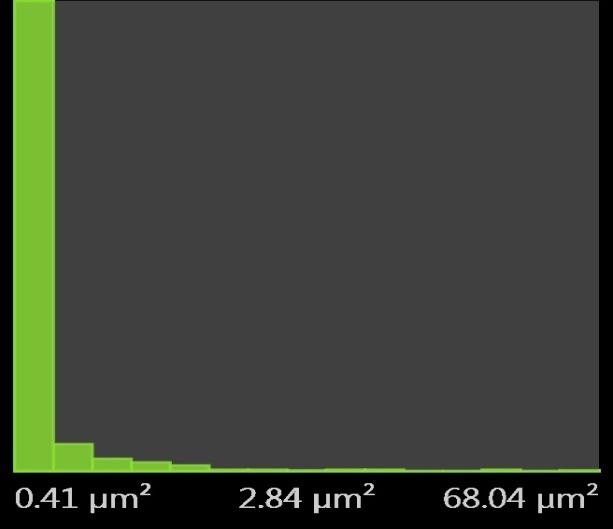 | Figure 10. Pores Histogram for a freshly polished mild steel before immersion |
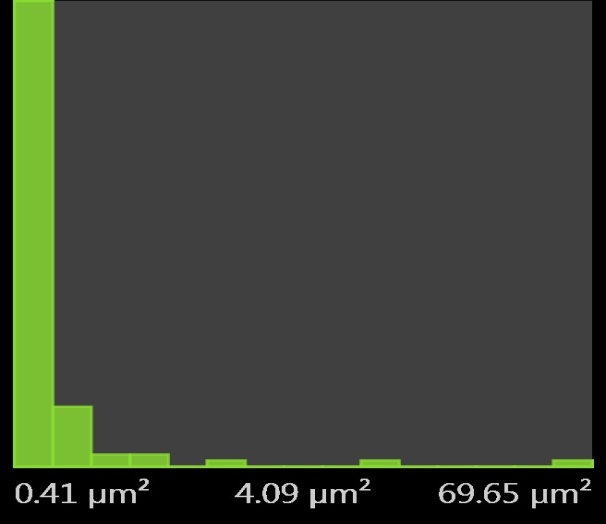 | Figure 11. Pores Histogram for 0.5M H2SO4 mild steel without inhibitor |
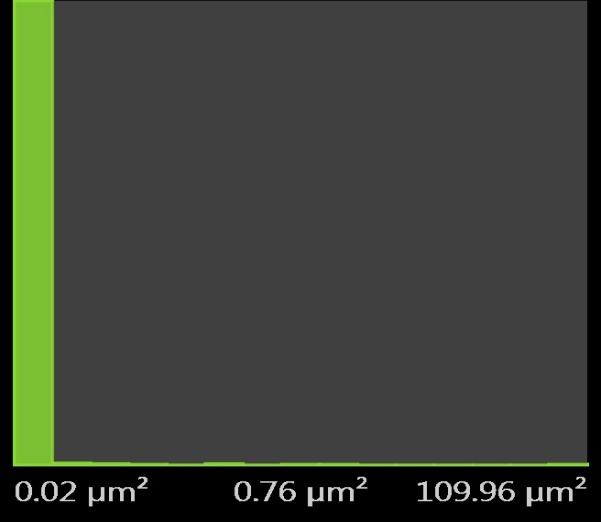 | Figure 12. Pores Histogram for 0.5M H2SO4 mild steel with inhibitor |
4. Conclusions
- This research investigated the phenomenon of corrosion mild steel metal in acidic media. Weight loss, thermometric measurements and Scanning electron microscope were used in this study. From the result and discussions in this thesis, the following conclusions were derived;• CTE acts as inhibitor for mild steel in 0.5M H2SO4 media.• Inhibition efficiency increases with increase in concentration of inhibitor.• Compared to several inhibitors in literature, CTE exhibited higher inhibition efficiency for Mild Steel.• The inhibition mechanism is explained by adsorption. The adsorption of CTE inhibitor in the acid corrodent obey Langmuir adsorption isotherm.• The thermodynamic parameters calculated from the adsorption isotherms showed that strong electrostatic interaction between the mild steel surface and CTE (physisorption) occurred in the inhibition process.• Thermometric measurements data show that the CTE acted as inhibitor in 0.5M H2SO4 for mild steel corrosion.• The rates of mild steel corrosion generally are increased with increasing temperature.• Corrosion rate decreased with CTE addition in 0.5M H2SO4 and protection efficiency reaches to 97.3141% by weight loss method and to 90% by thermometric method.• Thermometric measurements data show that the reaction number decreased with CTE addition to 0.5M H2SO4 and protection efficiency increased with increase in CTE concentration.• The metallographic micrographs showed that the morphology pits of mild steel in the absence of CTE demonstrated a higher number than that in the presence of inhibitor.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge with deep gratitude, the contributions of the academic staff of Chemistry Department, Nigeria Defence Academy, NDA Kaduna towards the actualisation of this great work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML