-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2015; 5(3): 54-63
doi:10.5923/j.ijmc.20150503.02
Modified Chitosan for Efficient Dye Adsorption in Low Acid Media
Nehal A. Salahuddin, Mohamad M. Ayad, Magda E. Essa
Department of Chemistry, Faculty of Science, Tanta University, Tanta, Egypt
Correspondence to: Nehal A. Salahuddin, Department of Chemistry, Faculty of Science, Tanta University, Tanta, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Polyoxypropylenediamine-modified chitosan (D2000-Cs) was prepared by crosslinking of Cs by using epichlorohydrin (ECH) followed by chemical modification using D2000. D2000-Cs was characterized by X-ray diffraction (XRD), Fourier transform infrared (FT-IR), scanning electron microscopy (SEM), and thermal gravimetric analysis (TGA). The stability of D2000-Cs in acid medium was also tested. The adsorption of an anionic dye, acid green 25 (AG) from acidic aqueous solution onto D2000-Cs was investigated. The adsorption experiments were conducted by varying parameters namely: initial concentration of AG, pH and temperature. The kinetic models: pseudo-first order, pseudo-second order, and the intraparticle diffusion models were applied to the experimental data. The adsorption process was found to follow pseudo-second order kinetic model. The Langmuir, Temkin and Freundlich models were used to fit the adsorption isotherms. It was observed that the experimental data fits very well to the Langmuir model.
Keywords: Polyoxypropylenediamine, Chitosan, Acid Green 25, Adsorption isotherm, Kinetics
Cite this paper: Nehal A. Salahuddin, Mohamad M. Ayad, Magda E. Essa, Modified Chitosan for Efficient Dye Adsorption in Low Acid Media, International Journal of Materials and Chemistry, Vol. 5 No. 3, 2015, pp. 54-63. doi: 10.5923/j.ijmc.20150503.02.
Article Outline
1. Introduction
- Colored organic effluent is produced in different industries such as textile, rubber, paper, plastic and cosmetics. The colored effluents of wastewater from these industries can be mixed in surface and ground water systems, and may transfer to drinking water and bring a chief threat to the human health due to their toxic or mutagenic and carcinogenic effects [1, 2]. Therefore, colored wastewater cannot be discharged without adequate treatment and dye removal. Physico-chemical processes such as coagulation and flocculation [3], membrane separation [4], oxidation [5], electro-coagulation [6], and adsorption on activated carbon and clays [7] were employed for the treatment of dye-containing wastewater. Among these methods, adsorption process is considered to be promising technology. Activated carbon is the most widely used commercial adsorbent due to its excellent adsorption capacity [8]. However, the high cost and the poor regeneration are the main drawbacks for the use of activated carbon as adsorbent [9]. Many studies were undertaken to find low cost sorbents such as cotton, fly ash, guava leaf powder, sugarcane bagasse pith, rice husk, and coconut coir [10-16].Recently, most researchers concentrated towards natural polymeric materials, because they are renewable, biodegradable, non-toxic, and hence they are considered environment friendly materials [17]. Among the natural polymeric materials, chitosan (Cs) is the most attractive polymer due to its low cost and availability. Cs is a linear copolymer composed of (1–4)-linked D-glucosamine and N-acetyl-D-glucosamine and it is obtained by alkaline deacetylation of chitin (the second abundant polymer in nature after cellulose) [18]. Cs was widely used for the removal of heavy and transition metals [8, 19, 20] due to its high number of functional amines and hydroxyl groups, thus enabling the adsorption of anionic dyes through electrostatic attraction. However, Cs is soluble in dilute organic acids and is difficult to be separated from the solution using traditional separation methods such as filtration and sedimentation. To overcome such a problem, various physical and chemical modifications were developed to improve the chemical stability of Cs in acidic media. Recently, many researchers were interested in the chemical modification of Cs by cross-linking method using cross-linking agents such as glutaraldehyde [21, 22], epichlorohydrin (ECH) [23, 24] and ethyleneglycol diglycidyl ether [25, 26]. Although the crosslinking methods were found to enhance the resistance of Cs against acids, it reduced the adsorption capacity of Cs. To overcome this problem, the crosslinked Cs can be modified with Polyoxyalkylene diamines to increase the adsorption capacity. The aim of the present work is directed to prepare a crosslinked Cs followed by modification with polyoxypropylenediamine (D2000). Resultant D2000-Cs has the advantage of improving the chemical stability of Cs in acidic media. D2000-Cs was characterized using by X-ray diffraction (XRD), Fourier transform infrared (FT-IR), scanning electron microscopy (SEM) and thermal gravimetric analysis (TGA). Afterward, D2000-Cs was tested for the adsorption of anionic dye, acid green 25 (AG) from acidic aqueous solutions. The effect of physicochemical parameters, that influence the adsorption process such as the initial concentration of AG, pH and temperature, were also investigated.
2. Experimental
2.1. Materials
- Cs with a molecular weight of 100,000-300,000 and 90% degree of deacetylation (Acros, USA), ECH 99% (Acros, USA), Polyoxypropylene-diamine (D2000) with molecular weight of 2000, primary amine content is 0.97 meq g-1 (Huntsman corporation TX, USA) were used without further purification. AG (Aldrich, UK), acetic acid and methanol (Adwic, EGYPT) and extra pure NaOH pellets (Lobachemie, INDIA) were used as received.
2.2. Preparation of D2000-Modified Cs (D2000-Cs)
- The preparation of D2000-modified Cs (D2000-Cs) was carried out by the following steps:
2.2.1. Preparation of Cs Gel
- The Cs gel was prepared using the previously reported method by Huang et al. [27]. Accordingly, a solution of Cs was prepared by dissolving 1 g of Cs in 33.3 ml of 2% acetic acid followed by drop wise addition of Cs solution to an alkaline precipitation bath (50 mL of 0.5 M NaOH aqueous solution). Afterward, the gelatinous precipitate of Cs was collected and washed for several times by distilled water then by ethanol to remove residual NaOH. Finally, the precipitate was stored in distilled water for further use.
2.2.2. Preparation of Crosslinked Cs
- The Cs gel obtained from the previous step was suspended in 100 mL of a mixture of water–methanol (v/v, 1:1). Afterward, an excess amount of ECH (2 mL) was added drop wise to the suspension solution then the mixture was stirred at 60 °C for 24 h and cooled. Finally, the product was filtered and washed three times by water and ethanol to give crosslinked Cs (ECH-Cs) with nitrogen content 1.24 wt%.
2.2.3. Preparation of D2000-Modified Cs (D2000-Cs)
- D2000-Cs was prepared by suspending crosslinked Cs in 100 mL methanol/water mixture (1:1, v/v) followed by addition of 7.63 g D2000. The reaction mixture was kept at 60°C with stirring for 24 h. Then, the product was filtered, washed three times by water and ethanol to remove the unreacted D2000 and dried at 60°C. The product was finally grinded (Nitrogen content 4.31wt %).
2.3. Solubility Test
- The solubility of D2000-Cs was examined in acidic medium by adding 5 mg of the sample into 10 mL HCl (pH 2.3) for 24 h then the sample was reweighed.
2.4. Swelling Test
- The D2000-Cs sample was weighed in a sintered glass, Wₒ and then immersed in buffer solution (pH = 3, 5.4 and 7.8) at 25°C. After immersion, the sample was centrifuged and reweighed immediately, Wt. The process was repeated until Wt becomes constant. The swelling degree of the new material expressed as the amount of adsorbed buffer per 100 g of dry sample, the swellability percentage was calculated using the formula:
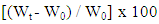 | (1) |
2.5. Adsorption Batch Experiments
- A stock solution of AG dye (186.8 mg L-1) was prepared in freshly distilled water, and the solutions of desired concentrations (18, 23, 28, 31 and 36 mg L-1) were obtained by successive dilution. Calibration curve was constructed by measuring the absorbance of the AG solutions at λmax = 642 nm.Adsorption experiments were carried out using 0.02 g of D2000-Cs and ECH-Cs as adsorbents. For each experiment, 50 mL of AG dye solution (18 mg L-1) along with the adsorbent were stirred magnetically (400 rpm) for 2, 5, 10, 15, 20, 25, 30, 35, 40, 50, 60 min at 25°C. The pH of the dye solution was adjusted to 3 for all the adsorption kinetic studies and equilibrium adsorption studies using 0.1 M HCl or 0.1 M NaOH. The samples of the dye solution, during the course of adsorption, were collected at regular intervals of time and centrifuged to remove the adsorbent. The absorption spectra of dye solutions before and after adsorption were recorded in the range of 750 – 500 nm. The concentration of dye solutions were determined at a wavelength of λmax = 642 nm. The quantity of adsorbed dye Qe (mg g-1) and the removal efficiency (R%) were calculated from the following equations:
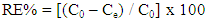 | (2) |
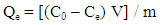 | (3) |
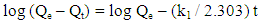 | (4) |
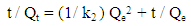 | (5) |
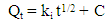 | (6) |
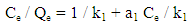 | (7) |
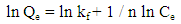 | (8) |
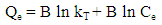 | (9) |
2.6. Instruments
- The UV visible absorption spectra were measured using spectrometer, UVD-2960 (Labomed. INC). Fourier-transform infrared (FT-IR) spectra were recorded using Bruker, Tenson 27 FTIR spectrophotometer with frequency range of 4000 – 400 cm−1 using KBr disk technique. X-ray diffraction (XRD) analysis was measured by GNR, APD 2000 PRO step scan X-ray diffractometer, Cu Kα radiation (l.54 Å), (40 KV and 30 mA) at a step per second of 0.02°. Thermal gravimetric analysis (TGA) was carried out using Perkin Elmer, STA 6000. The morphology of crosslinked Cs and D2000-Cs was studied by scanning electron microscope (SEM JEOL, JSM-5200LV) at an acceleration voltage of 25 KV. Quantitative elemental analysis for N in crosslinked Cs, D2000-Cs was performed on Heraeus (microanalysis center, Cairo University, Giza, Egypt).
3. Results and Discussion
3.1. Synthesis and Characterization of D2000-Cs
- The aim of the present work is to produce a new material with high adsorption capacity and chemical stability in acid medium. To achieve this, Cs was dissolved in acetic acid to yield hydrogel in water. After that, Cs beads were crosslinked by using excess amount of ECH. Finally, the crosslinked Cs was modified by D2000. Similar modification was reported using ethylenediamine [27]. The prepared D2000-Cs showed satisfactory chemical stability in acid medium which was confirmed by its insolubility in strong acid media (pH = 2.3), however, Cs was completely soluble in weak acid medium.The FT-IR spectrum of Cs (Figure. 1 (a)) shows a band at 3443 cm-1 due to the axial stretching vibration of OH superimposed to NH2 stretching and inter- and intramolecular hydrogen bonding of Cs. The bands at 2921 and 2878 cm-1 are due to C–H asymmetric stretching vibration of CH2 and C–H symmetric stretching vibration of CH3 groups, respectively [36]. The band at 1646 cm-1 is assigned to C=O stretching of amide group (NHCOCH3) due to partial deacetylation of chitin. The bands at 1603, 1460 and 1426 cm-1 are attributed to bending vibration of NH2, CH2, CH3, respectively. The sharp peak at 1384 cm-1 is related to C–N stretch of amide group and CH3 bending. The band at 1325 cm-1 refers to CH2 scissoring and OH bending vibration 29. The bands at 1259 and 1155cm-1 are due to C–N and the bridge C–O–C stretching vibrations, respectively, while the peaks at 1082 and 1028 cm-1 refer to C–O and CH2OH stretching vibrations, respectively. The peak at 896 cm-1 is attributed to wagging of saccharine structure of Cs [37].The FT-IR spectrum of the ECH-Cs, Figure. 1 (b) shows all the significant characteristic bands of Cs with some shifts in the position of bands. The same functional groups of the ECH were present in the Cs. Therefore, the same vibrations were observed, but with different intensities. The peak at 1646 cm-1 related to C=O stretch of amide group shifted to 1639 cm-1. In addition, the band at 1603 cm-1 of NH2 group disappeared which is a spectroscopic evidence for the covalent bonds formation between ECH and NH2 groups of Cs. The peak at 1259 cm-1 related to C-N stretch became weak and shifted to 1255 cm-1 proving the introduction reaction of ECH on NH2 position of Cs [37].Significant changes were observed in the spectrum of D2000-Cs presented in Figure. 1 (c). A band at 1603 cm-1 that is attributed to NH bending in primary amine groups (NH2) was observed. The band at 2921 cm-1 related to asymmetric stretching vibration of C-H of CH2 group shifted to 2973 cm-1 due to the insertion of D2000 into Cs. In addition, the bands at 1460 and 1384 cm-1 that are related to CH2 bending and C–N stretching vibrations of amide group became more intense and shifted to 1456 and 1375 cm-1, respectively. Furthermore, two new peaks appeared at 1301 and 1261 cm-1 due to the primary and secondary amino groups, which verify the grafting of D2000.
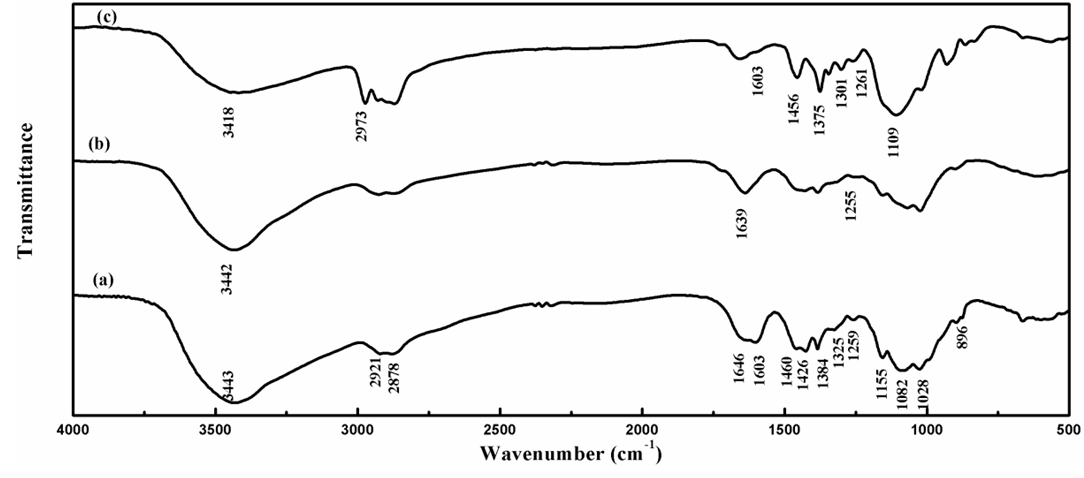 | Figure 1. FTIR spectra of Cs (a), ECH-Cs (b) and D2000-Cs (c) |
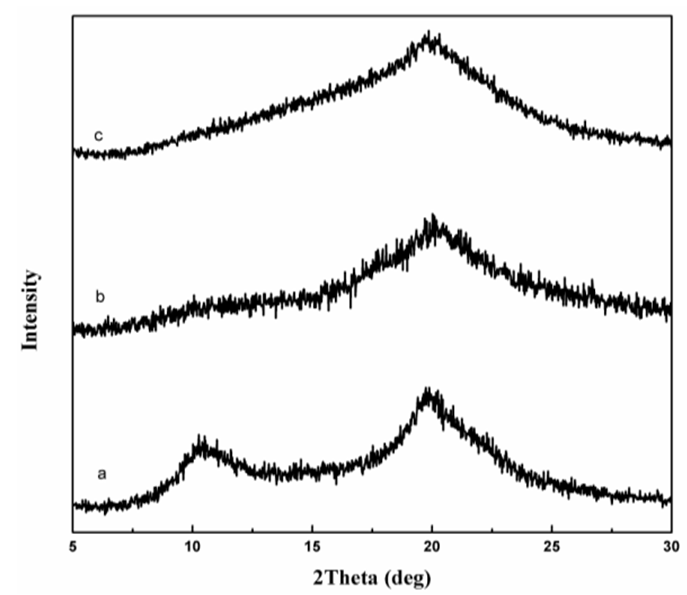 | Figure 2. XRD spectra of Cs (a), ECH- Cs (b) and D2000-Cs (c) |
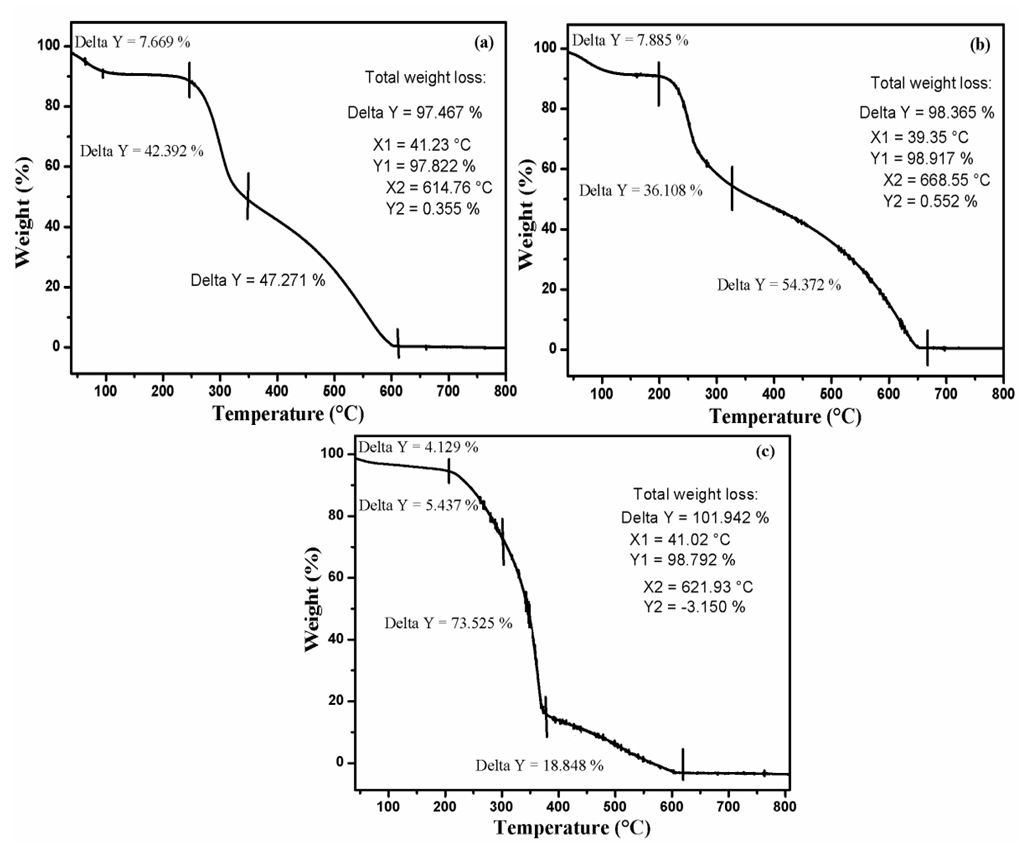 | Figure 3. TGA of Cs (a), ECH- Cs (b) and D2000-Cs (c) |
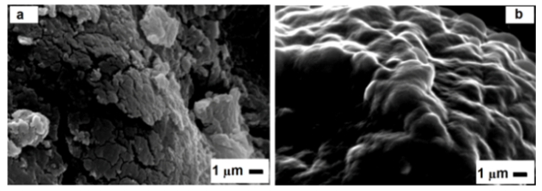 | Figure 4. SEM of ECH- Cs (a) and D2000-Cs (b) |
3.2. Adsorption of AG onto D2000-Cs
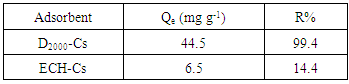
- The D2000-Cs can be utilized as an ideal material for acidic wastewater treatment that includes anionic dyes. To investigate the effect of crosslinking and modification by D2000 on the adsorption of dyes, experiments were conducted for adsorption at pH = 3 from 50 mL of 18 mg L-1AG with 20 mg of ECH-Cs and D2000-Cs. Figure. 5 shows the adsorption capacity against time using D2000-Cs and ECH-Cs. It is clear that D2000-Cs exhibited much higher adsorption capacity as compared to that of crosslinked Cs. The amount of dye adsorbed per unit mass (Qe) of D2000-Cs and crosslinked Cs were, 44.5 and 6.5 mg g-1 with adsorbtivity (R%) equals 99.4 and 14.4%, respectively showed in table 1. This may be attributed to the high surface area of D2000-Cs and the large number of binding sites (NH2 groups), which lead to high interaction between AG and the D2000-Cs. In addition, the loading of D2000 improves the adsorptivity. Amine groups can significantly uptake the AG in acid medium through electrostatic interaction between NH3+ and O- in AG.
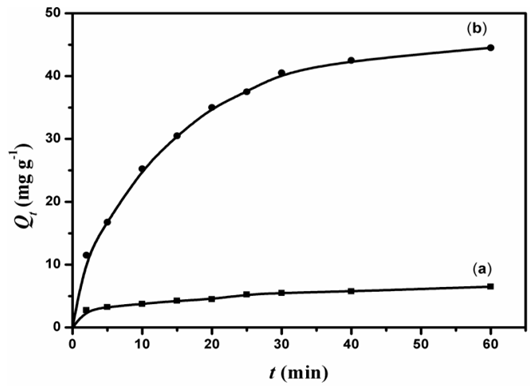 | Figure 5. Adsorption capacity of ECH- Cs (a) and D2000-Cs (b) |
|
3.3. Adsorption Kinetics
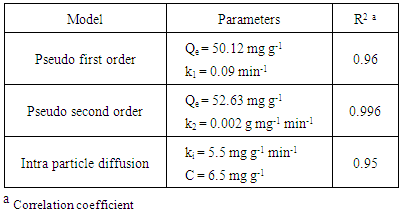
- The kinetic analysis, in order to evaluate the kinetic mechanism that controls the adsorption process, were tested by applying the pseudo first order [28], pseudo second order [29, 30] and intraparticle diffusion [31]. Figure. 6 (a-c) shows the corresponding linear plots. The parameters, k1, Qe , K2 and the correlation coefficient (R2) were determined and presented in Table 2. The results of the regression analysis showed that the adsorption of AG dye onto D2000-Cs was best described by pseudo-second order with the highest correlation coefficients.
|
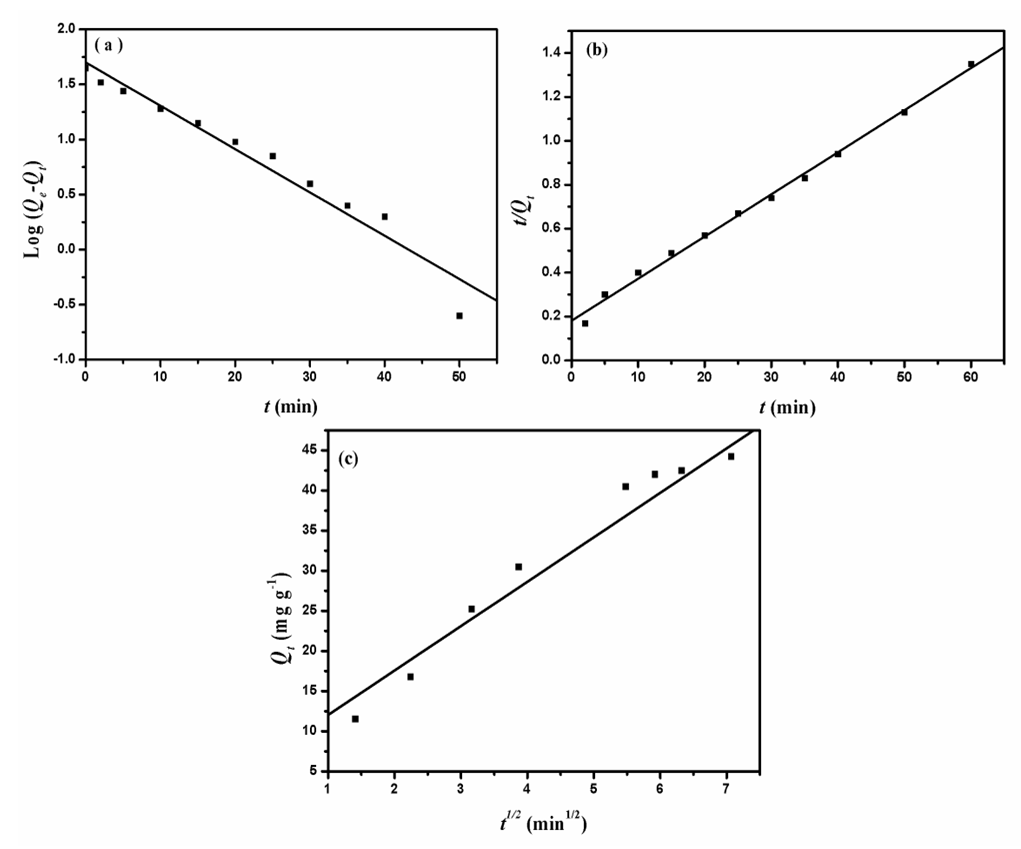 | Figure 6. The pseudo first order (a), pseudo-second-order (b) and Intraparticle diffusion kinetic plots for adsorption of AG onto D2000-Cs |
3.4. Adsorption Isotherms
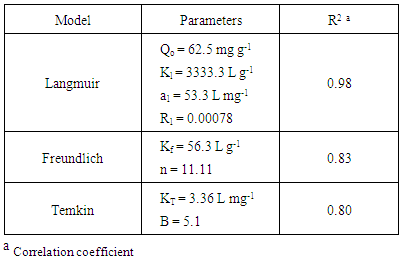
- The adsorption isotherms of the adsorption of AG into D2000-Cs were studied. The most common sorption models used to fit the experimental data are Langmuir [33], Freundlich [34], and Temkin [35], are showed in Figure. 7 and the correlation coefficient (R2) was calculated and reported in Table 3. For the three studied systems, R2 of Langmuir isotherm is more near to unity and is better than R2 of Freundlich and Temkin isotherms. Hence, the Langmuir model is the most widely used for the sorption of AG onto D2000-Cs.
|
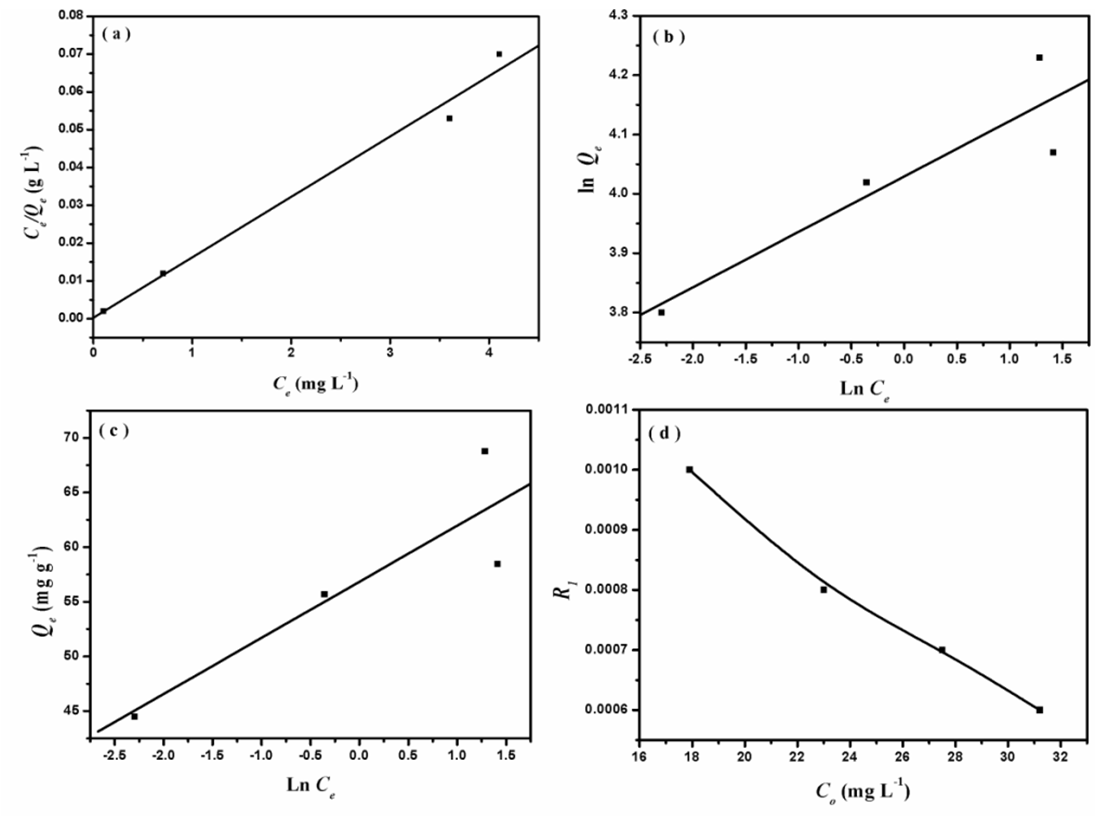 | Figure 7. Langmuir isotherm model (a), Freundlich isotherm model (b), Tempkin isotherm model (c) and separation factor versus initial AG concentration (d) |
3.5. The Effect of pH
- The pH of the dye solution plays an important role in the whole adsorption process, particularly affecting the adsorption capacity. Figure. 8 shows that the amount of dye adsorbed onto D2000-Cs increased with decreasing pH of solution. At lower pH, more protons will be available for the protonation of NH2 groups in D2000 or Cs leads to increasing the electrostatic attraction force between D2000-Cs and dye molecules. At high pH, the protonation of the amino groups decreases and leads to decrease adsorption [44]. The values of Qe for the adsorption of AG dye solution onto D2000-Cs at pH = 3, 4.5, 7 and 9 were 40.5, 32, 28 and 16 mg g-1, respectively.
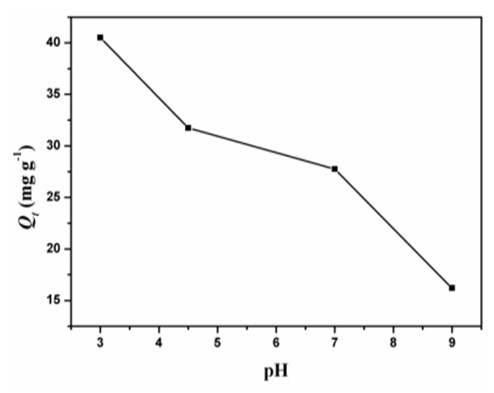 | Figure 8. Adsorption of AG onto D2000-Cs at different pH |
3.6. The Effect of Initial Dye Concentration
- Figure. 9 shows the effect of initial dye concentration (Co) on the adsorption kinetics of the D2000-Cs. The initial pH of dye solution was adjusted to 3. The values of Qe for the adsorption of AG dye solution (50 ml) with concentrations 18, 23, 31 and 36 mg L-1 using D2000-Cs (0.02 mg) are 44.5, 55.4, 69 and 84 mg g-1, respectively. Figure. 9 shows that the adsorption capacity increases with increasing Co.
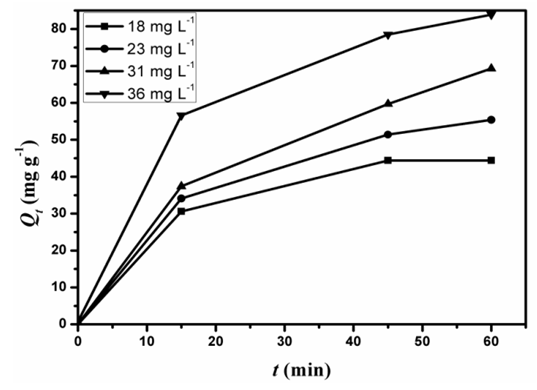 | Figure 9. Effect of initial concentration on the adsorption of AG by D2000-Cs |
3.7. The Effect of Temperature
- The effect of temperature on the adsorption capacity of AG onto D2000-Cs was studied at pH = 3 and the results are shown in Figure. 10. The adsorption capacity increased with increasing temperature from 25 to 50°C, which indicates the endothermic nature of the adsorption process.
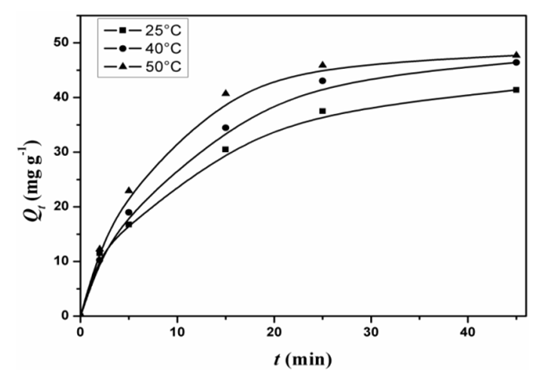 | Figure 10. Effect of temperature on the adsorption of AG onto the D2000-Cs |
4. Conclusions
- Cs was modified by using polyoxypropylenediamine (D2000) to obtain (D2000-Cs). The product was tested for the adsorption of anionic dye, acid green 25 (AG), from acidic aqueous solutions and shows good stability. The adsorption ability of D2000-Cs for removal of anionic dyes in acid medium is efficient and overcomes the drawbacks of using Cs as adsorbent due to its solubility in acid medium. The initial concentration of AG, pH and temperature significantly affect the adsorption process and the adsorption capacity of D2000-Cs. The Langmuir model agrees very well with the equilibrium isotherm and the pseudo-second order describe the equilibrium kinetics.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML