-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2015; 5(1): 14-23
doi:10.5923/j.ijmc.20150501.03
Inhibition of Mild Steel and Aluminium Corrosion in 1M H2SO4 by Leaves Extract of African Breadfruit
Ejikeme P. M.1, 2, Umana S. G.1, Menkiti M. C.3, 4, Onukwuli O. D.2
1Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka
2Department of Chemistry, University of Pretoria, Pretoria, South Africa
3Department of Chemical Engineering, Nnamdi Azikiwe University, Awka
4Water Resources Centre, Texas Tech University, Lubbock, Texas, U.S.A
Correspondence to: Ejikeme P. M., Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Inhibitory effects of African breadfruit leaves extract (BLE) on the corrosion of mild steel and aluminium in 1M sulphuric acid (H2SO4) solution was studied at 30℃ and 60℃. Kinetic, thermodynamic and adsorption parameters for the inhibition process were determined from the adsorption data. Corrosion rates were found to increase as temperature increased, but decreased in the presence of the extract compared to the blank. Inhibition efficiency increased with increase in inhibitor concentration reaching 70.55% for mild steel and 78.56% for Al at 30°C in the presence of 5g/L of BLE. The results obtained also show that the plant extract inhibited the corrosion process by physical adsorption mechanism and the process followed Freundlich adsorption isotherm model better than Langmuir and Temkin models. Kinetic analysis showed that the equilibrium constants were higher for mild steel (1.136) than aluminium (0.985), at 30℃. The implications of these results were discussed for the plant extract.
Keywords: African breadfruit, Surface coverage, Activation energy, Adsorption isotherms, Activation energy
Cite this paper: Ejikeme P. M., Umana S. G., Menkiti M. C., Onukwuli O. D., Inhibition of Mild Steel and Aluminium Corrosion in 1M H2SO4 by Leaves Extract of African Breadfruit, International Journal of Materials and Chemistry, Vol. 5 No. 1, 2015, pp. 14-23. doi: 10.5923/j.ijmc.20150501.03.
Article Outline
1. Introduction
- Mineral acids are widely used in various industries for pickling of metals at temperatures up to 60°C. This technique besides being used to remove corrosion scales from steel surfaces without causing acid attack of the bulk metal is also effectively applied in cleaning of industrial equipment and acidization of oil wells. Corrosion of oil equipment is of significant cost, which is additional to mechanical and other operational problems that require work-over and repairs. It is therefore imperative to add corrosion inhibitors to the solution during pickling in other to reduce the degree of metal attack and the rate of acid consumption (Umoren et al., 2008; Loto et al., 2014).Corrosion can be viewed as the process of returning a metal to their natural state, i.e. the ores from which they were originally obtained. Approximately one-fifth of the iron and steel produced annually is used to replace rusted metal (Zumdahl, 1993). Mild steel and aluminium are used in fabricating various reaction vessels, reaction tanks, and pipes etc, for industrial uses due to their availability and low cost. However when used in aggressive media they tend to corrode which causes severe loss and malfunctioning of industrial equipment. Organic and inorganic compounds such as indigo dyes (Oguzie et al., 2004), methyl red (Ebenso, 2004a,b), have been used in recent past as corrosion inhibitors. However, discharge of such materials has become unacceptable due to environmental hazards (Sharmila et al., 2010). Plants extracts known for their biodegradability, eco-friendliness, cost effectiveness, low toxicity and easy availability are considered green inhibitors (Sethuraman et al., 2005). The recent trend is towards environmentally friendly and organic inhibitors. Accordingly, great research efforts have been directed towards formulating these inhibitors. Some of the plant extracts that have been studied include Gum acacia (Mobin and Khan, 2013), Azadirachta indica and Hibiscus Sabdariffa (Oguzie, 2008), ethanol extract of Vernonia amygdalina (Odiongenyi et al., 2009), Telfaria occidentalis (Oguzie, 2005), Allium sativum (Okafor et al., 2005), and ethanol extract of Musa acuminata peel (Eddy et al., 2008), Phyllanthus amarus leave (Okafor et al., 2008), aqueous extract of Fenugreek leaves (Noor, 2007), Calendula Officinalis flower (Subha et al., 1999), aqueous extract of olive leaves (El-Etre, 2007), Berberine (Yan Li et al., 2005) and Damsissa (Abdel-Gaber, 2008) have been studied. Also, Sansevieria trifasciata (Oguzie, 2007), Aloe plant (Al-Turkustani et al., 2010), Bauhinia purpurea leaves (Patel et al., 2009), bamboo leaf extract (Li et al., 2014), Camellia Sinensis (Loto et al., 2014) and red onion skin acetone extract (James and Akaranta, 2009) investigation as corrosion inhibitors have been carried out. Despite the availability and variety of plant materials only few have been thoroughly investigated and data obtained for various corrodents.African breadfruit (Treculia Africana Decne) is a tropical forest tree that belongs to the plant family Moraceae. The tree plant has beautiful shape and makes a good shade arising from its broad leaves. It bears big heavily seeded oval fruit heads of mass 2.5–13.62 kg and attains 45 cm in diameter, yielding 5–15 kg edible cooking oil seeds after processing. The tree is evergreen, grows up to 40 m high, and produces 30–50 fruits annually (Akubor, 1997; Nwokocha and Ogunmola 2005). The fruits and leaves are good fodder for goats, sheep and pigs (Etukudoh, 2003). To the best of knowledge of the authors, the only literature on the use of African breadfruit leave extract as corrosion inhibitor is our earlier study (Ejikeme et al., 2012), in which we examined the inhibitive effect of the plant extract on the corrosion of aluminium in HCl solution. The results of the said study encouraged us to investigate further the corrosion inhibition of the plant extract in a more aggressive 1M H2SO4 solution on both aluminium and mild steel. This comparative study we speculate will help to access the applicability or otherwise of the plant extract as corrosion inhibitors in the two widely used construction materials.
2. Materials and Methods
2.1. Materials
- The composition of the mild steel is as follows: 0.21% C, 0.38% Si, 0.09% p, 0.01% Al, 0.05% Mn, 0.05% S and the remainder iron. The aluminium was purchased at System Metals Industries Limited, Calabar, Nigeria. It was of 98% purity and of the type AA 1060. Other materials and apparatus used include: rotary evaporator, digital high precision weighing balance (Metler), UV-visible spectrophotometer (Sperctron 21D) PEC Medicals, U.S.A. All reagents used were of analytical grade unless otherwise stated.
2.2. Preparation of African Breadfruit Extract
- The leaves of African breadfruit were obtained from Nsukka, Southern Nigeria. The leaves were sun-dried and crushed to powder using a manual grinding machine. About 300g of the ground leaves was weighed and soaked in 96% ethanol. After 48h they were filtered and the filtrate concentrated to paste using rotary evaporator. Various weights of the inhibitor ranging from 0.1g - 0.5g were weighed and dissolved in 1M H2SO4.
2.3. Phytochemical Screening
- The phytochemical analysis of the extract was by standard methods as described in Evans (2000) and Harbone (1998). Specifically the extract was screened for phytochemicals that included tannins, flavonoids, alkaloid, terpenes, saponins, phlobatannins, deoxy sugar, and cardiac glycoside.
2.4. Gravimetric Corrosion Study Technique
- For the corrosion study, previously weighed metal coupons were placed in 100 ml of 1M H2SO4 solution (blank) and in 100 ml of 1M H2SO4 solution with the various concentrations of BLE ranging from 0.1 – 0.5g in open beakers labeled A, B, C, D, E. The beakers were placed in a thermostated paraffin oil bath maintained at 30℃ or 60℃ as the case may be. In each experiment, the clean metal coupons were suspended with the aid of glass rod and hook. These coupons were retrieved progressively at 2h intervals for 8h, washed, dried in acetone and re-weighed. The weight loss was taken as the difference in weight of the specimen before and after immersion determined by weighing with the digital balance. The tests were conducted in triplicate to guarantee the reliability of the results and the mean value of the weight loss was reported.
3. Results and Discussion
3.1. Phytochemical Screening
- The result of the phytochemical screening of the plant extract has been reported earlier (Ejikeme et al., 2012). Briefly, the plant extract contain a preponderance of alkaloids, terpenes, flavonoids, tannins, anthraquinones etc. Heteroatoms (P, O, N, S, Se) regarded as centers for adsorption (Obot et al., 2009) which abound in the phytochemicals of BLE are obviously involved in the adsorption of the extracts to the surface of the metals, inhibiting corrosion at such parts of the metal; the net effect of this process being the protection of the metal surface from the attack of the aggressive ions (SO42-) of the acid.
3.2. Corrosion Data
- Assessment of corrosion rate, inhibition efficiency and surface coverage for aluminium and mild steel with different inhibitor concentrations was carried out using Eqns. 1 to 3, respectively.
3.2.1. Corrosion Rate (χ)
- The corrosion rate (χ) was computed using Eqn. 1 as follows;
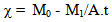 | (1) |
3.2.2. Inhibition Efficiency (%I)
- The inhibition efficiency (%I) was evaluated using Eqn. 2.
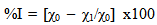 | (2) |
3.2.3. Surface Coverage (θ)
- The surface coverage (θ) of the inhibitor was obtained from the experimental data using Eqn. 3.
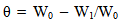 | (3) |
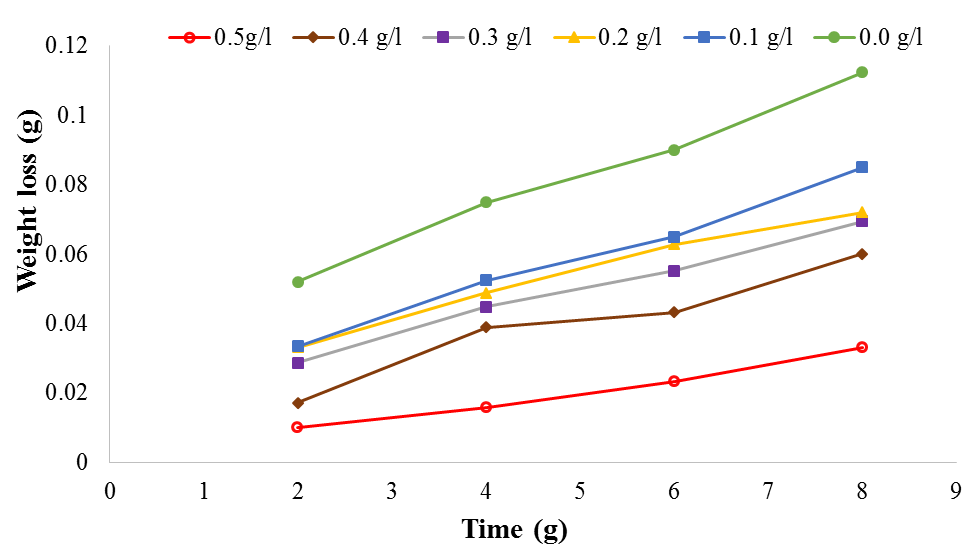 | Figure 1. Plot of weight loss against time for mild steel corrosion in 1M H2SO4 at 30℃ |
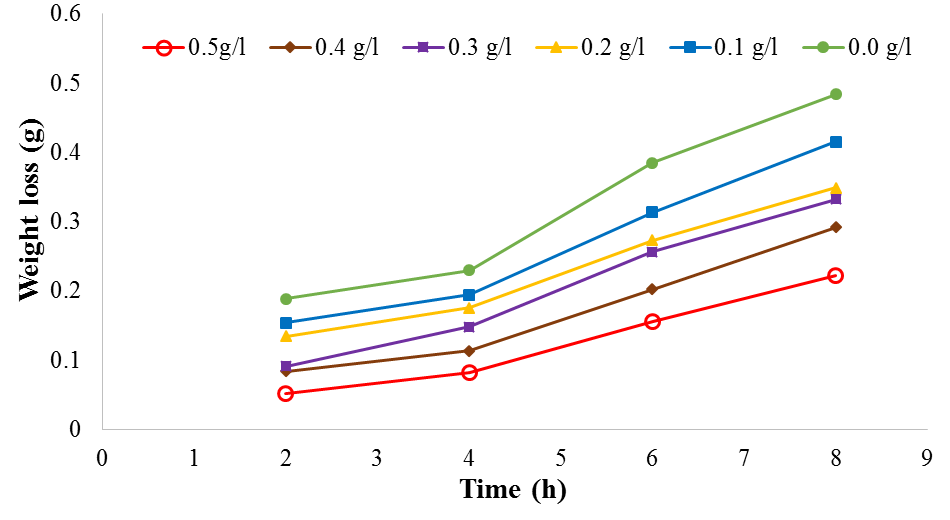 | Figure 2. Plot of weight loss against time for mild steel corrosion in 1M H2SO4 at 60℃ |
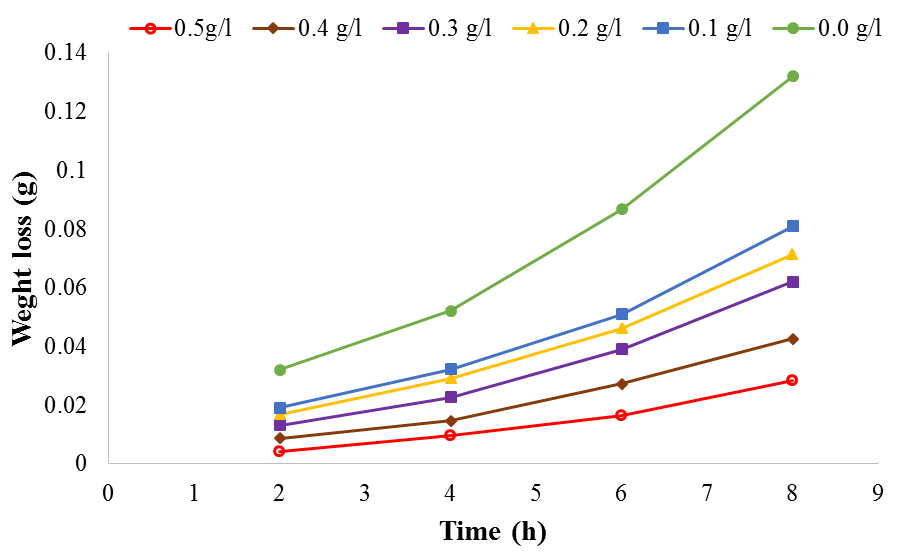 | Figure 3. Plot of weight loss against time for aluminium corrosion in 1M H2SO4 at 30℃ |
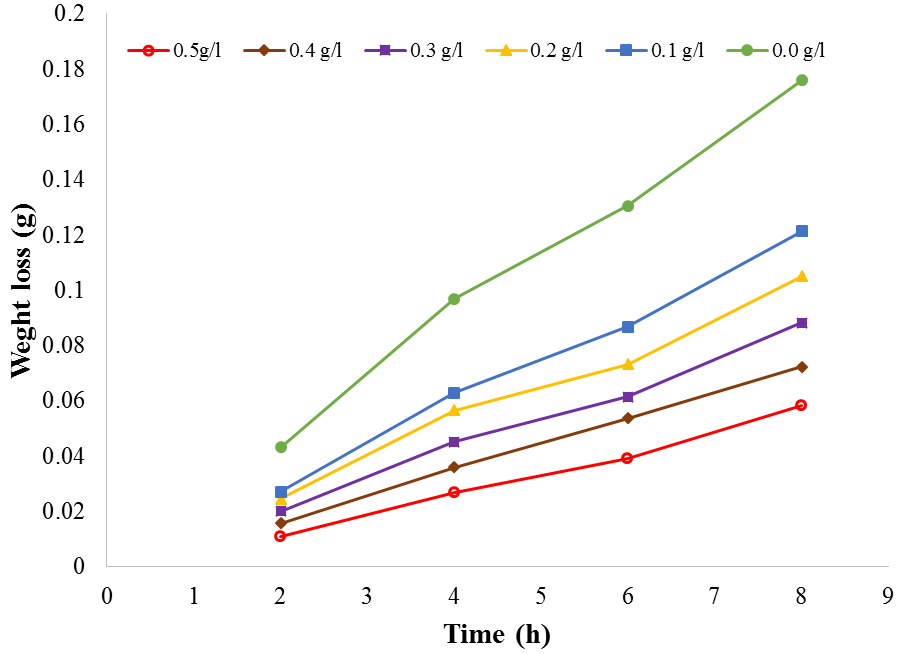 | Figure 4. Plot of weight loss against time for aluminium corrosion in 1M H2SO4 at 60℃ |
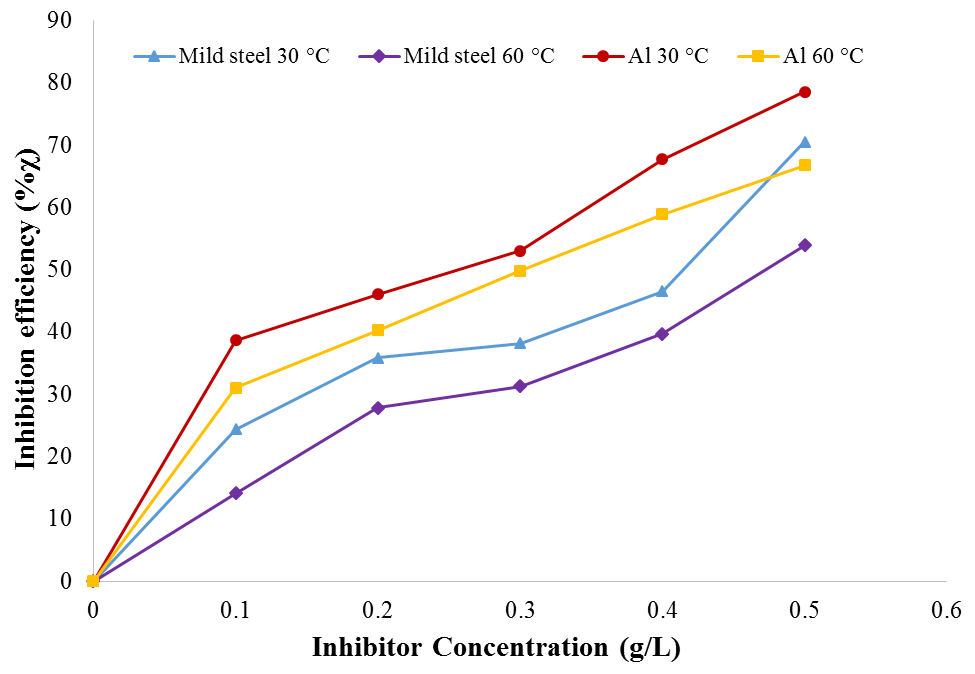 | Figure 5. Plot of inhibition efficiency of BLE for mild steel and Al corrosion in 1M H2SO4 |
3.3. Isotherm Study
- The mode and extent of the interaction between the BLE inhibitor and the metal surfaces were studied by applying adsorption isotherms. In isotherm studies, the linearity of the plot and good correlation coefficient may be interpreted to suggest that the experimental data for the studied inhibitor obey a particular adsorption isotherm but considerable deviation of the slope from unity shows that the isotherm may not be strictly applied. Temkin adsorption isotherm which assumes a uniform distribution of adsorption energy which increases with increase of the surface coverage is given by Eqn. 4 (Mu et al., 2005).
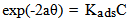 | (4) |
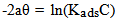 | (4a) |
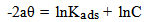 | (4b) |
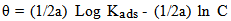 | (4c) |
| ||||||||||||||||||||||||||||||
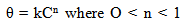 | (5) |
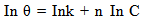 | (6) |
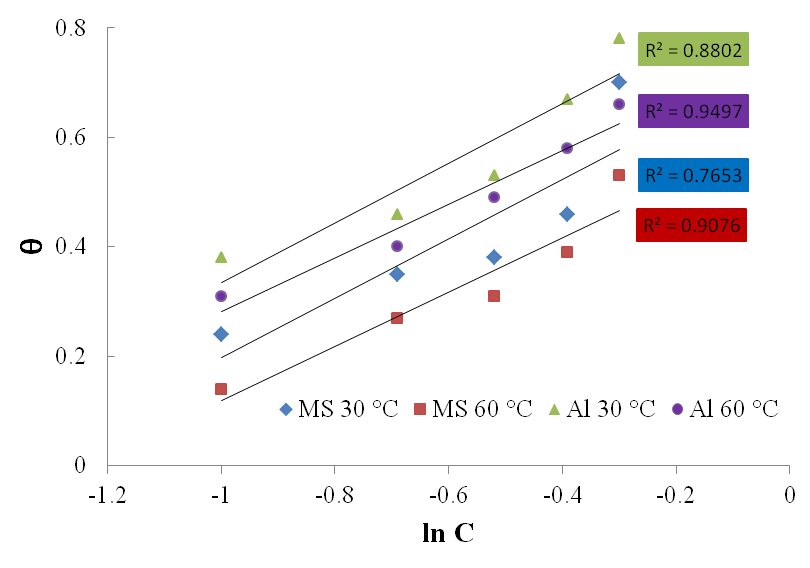 | Figure 6. Temkin adsorption isotherm for Al and mild steel corrosion in 1M H2SO4 at 30 and 60℃ |
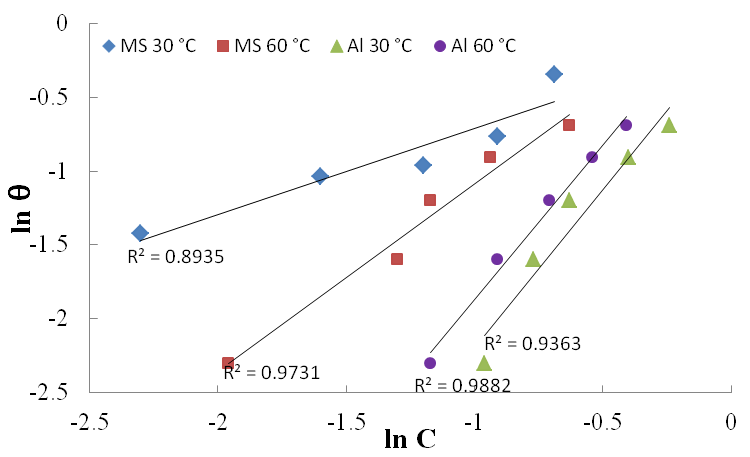 | Figure 7. Freundlich adsorption isotherm for Al and mild steel corrosion in 1M H2SO4 at 30 and 60℃ |
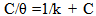 | (7) |
3.4. Thermodynamic and Kinetic Studies
- The values of ∆G°ads for the extract are given in Table 2. These values were found to be between -10.438 kJmol-1 and -10.645kJmol-1 for mild steel and -10.078 kJmol-1 and -10.795 kJmol-1 for aluminium which confirms that BLE adsorbed through physisorption on the aluminium and mild steel surfaces. The negative value of ∆G°ads indicates that the adsorptions of BLE unto the metal surfaces were spontaneous and that the layer adsorbed were stable (Bouklah, 2006). Values of ∆G°ads up to -20kJmol-1 like the ones obtained with BLE in this study are consistent with electrostatic interaction between the charged metal (which indicates physical adsorption) while those more negative than -40kJmol-1 involved charged sharing or transfer from the inhibitor molecule to the metal surface to form a co-ordinate type of bond (which indicates chemical adsorption) (Umoren et al., 2008). Overall, first order kinetic was better followed at lower temperatures for aluminium and vice–versa for mild steel. The values of the rate constant k, were evaluated from the plots of log ∆w against time.The value of activation energy (Ea) for the corrosion process in the absence and presence of the inhibitor were calculated using Arrhenius equation represented by Eqn. 8.
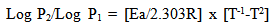 | (8) |
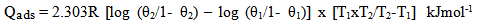 | (9) |
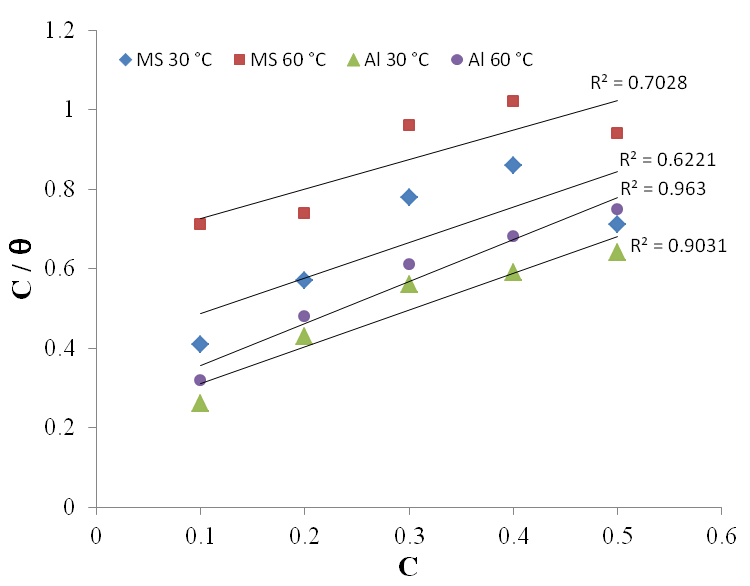 | Figure 8. Langmuir adsorption isotherm for Al corrosion in 1M H2SO4 at 30℃and 60℃ |
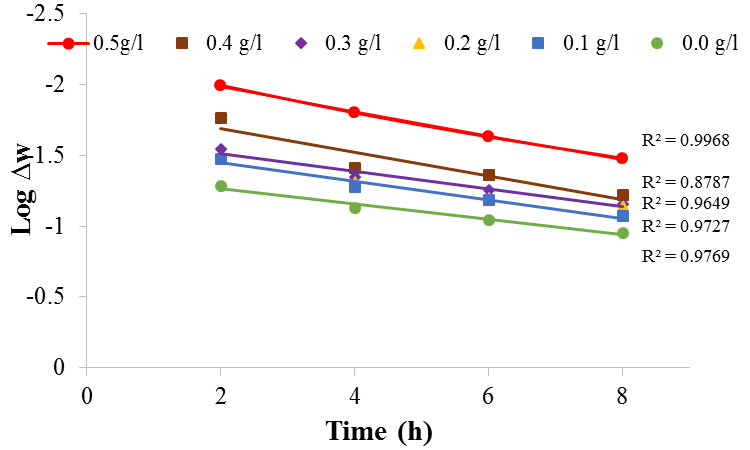 | Figure 9. Plot of log ∆W against time for mild steel in 1M H2SO4 in the absence and presence of BLE at 30℃ |
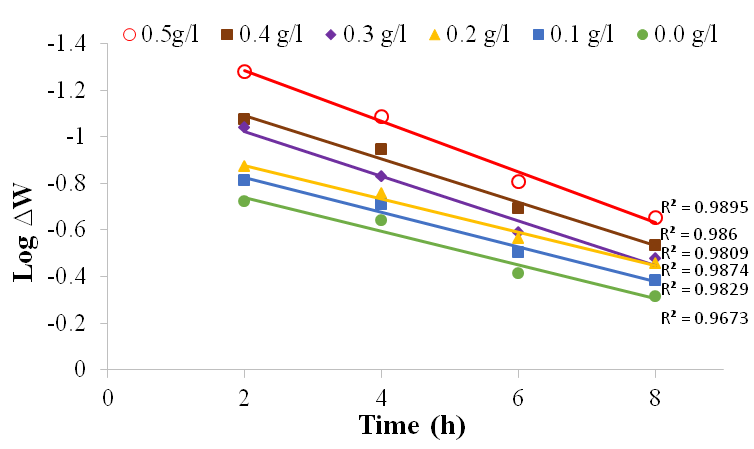 | Figure 10. Plot of log ∆W against time for mild steel in 1M H2SO4 in the absence and presence of BLE at 60℃ |
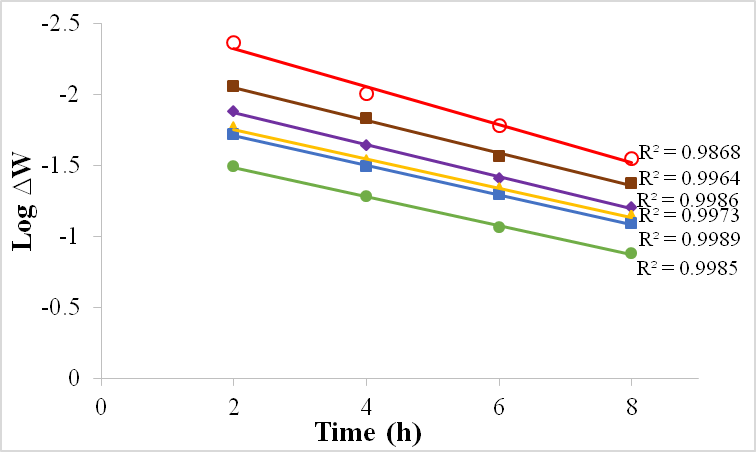 | Figure 11. Plot of log ∆W against time for Al in 1M H2SO4 in the absence and presence of BLE at 30℃ |
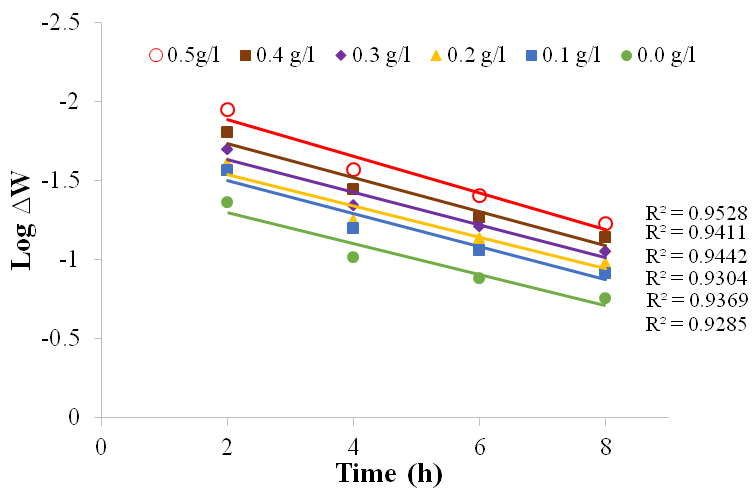 | Figure 12. Plot of log ∆W against time for Al in 1M H2SO4 in the absence and presence of BLE at 60℃ |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||
4. Conclusions
- African breadfruit leave extract acted as an inhibitor in the corrosion of mild steel and aluminium in H2SO4 solution. Corrosion rate was found to be greater in mild steel than aluminium and increased with increase in temperature. Inhibition efficiency increased with increase in the concentration of the extract but reduces with increase in temperature indicating that the adsorption of African breadfruit leave extract on the metals surfaces was unfavourable at high temperature. The adsorption of the extract on the metals surfaces obeyed Freundlich, Temkin and Langmuir adsorption isotherms at 30 and 60℃. Heat of adsorption was negative in all cases which show that adsorption and hence inhibition efficiency decreases with rise in temperature. Activation energies were higher in mild steel (40.28–52.46kJ/mol) than aluminium (7.46–19.75kJ/mol). Mechanism of physical adsorption is proposed for the adsorption process.
References
| [1] | Abdel-Gaber, A.M., Khamis, E., Abo-ElDahab, H., Adeel, S. 2008, inhibition of aluminum corrosion in alkaline solutions using natural compound, Mater. Chem. Phy. 109: 297-305. |
| [2] | Akubor, P.I. 1997, Proximate composition and selected functional properties of African breadfruit and sweet potato flour blends, Plant Foods Hum Nutr 51:53–60. |
| [3] | Al-Turkustani, A.M., Arab, S.T., Al-Dahiri, R.H. 2010, Aloe plant extracts as environmentally friendly inhibitor on the corrosion of aluminium in hydrochloric acid in absence and presence of iodide ions, Modern Appl. Sci., 4(5): 105 -124. |
| [4] | Bouklah, M., Hammouti, B. 2006, Thermodynamic characterization of steel corrosion for the corrosion inhibition of steel in sulphuric acid solutions by Artemisia. Portugaliae Electrochimica Acta 24: 457-468. |
| [5] | Ebenso, E.E. 2004a, Effect of halide ions on the corrosion inhibition of mild steel in H2SO4 using methyl red. Part 1. Bulletin of Electrochem., 19(5): 209-216. |
| [6] | Ebenso, E.E. 2004b, Effect of methyl red and halide ions on the corrosion of aluminium in H2SO4. Part 2. Bulletin of Electrochem., 20(12): 551-559. |
| [7] | Ebenso, E.E., Ibok, U.J., Ekpe, U.J., Umoren, S., Jackson, E., Abiola, O.K., Oforka, N.C., Martinez, S. 2004, Corrosion Inhibition studies of plant extracts on aluminum in acidic Medium. Trans. SAEST, 39: 117 -123. |
| [8] | Eddy, N.O., Odoemelam, S.A., Odiongenyi, A.O. 2008, Ethanol extract of Musa acuminate peel as an eco-friendly inhibitor for the corrosion of mild steel in H2S04. Am. Eur. Network Sci. Inform. 2(1): 35-42. |
| [9] | Ejikeme, P.M., Umana, S.G., Onukwuli, O.D. 2012, Corrosion inhibition of aluminium by Treculia Africana leaves extract in acid medium, Portugaliae Electrochim. Acta, 30(5): 317-328. |
| [10] | El-Etre, A.Y. 2007, Inhibition of acid corrosion of carbon steel using aqueous extract of olive leaves. J. Colloid Inter. Sci. 314: 578-583. |
| [11] | Etukudoh, I. (2003). Ethnobotany: Conventional and traditional use of plant. Vol. 1. The Verdict Press. Uyo Akwa Ibom State. |
| [12] | James A.O., Akaranta O. 2009, Corrosion inhibition of aluminium in 2.0 M hydrochloric acid solution by the acetone extract of red onion skin, Afr. J. Pure App. Chem. 3(12): 262-268. |
| [13] | Li, X., Deng, S., Fu, H. 2014, Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract, Corros. Sci. 62: 163-175. |
| [14] | Li, Y., Zhao, P., Liang, Q., Hou, B. 2005, Berberine as a natural source inhibitor for mild steel in IM H2SO4, Appl. Surf. Sci. 252: 1245-1253. |
| [15] | Loto, C.A., Joseph, O.O., Loto, R.T., Popoola A.P.I. 2014, Corrosion inhibitive behavior of Camellia Sinences on aluminium alloy in H2SO4, Int. J. Electrochem. Sci. 9: 1221-1231. |
| [16] | Mobin, M., Khan, M.A. 2013, Investigation on the adsorption and corrosion inhibition behavior of Gum acacia and synergistic surfactants additives on mild steel in 0.1 m H2SO4, J. Disper. Sci. Technol. DOI: 10.1080/01932691.2012. 751031. In Press. |
| [17] | Mu, G., Li, X., Liu, G. 2005, Synergistic inhibition between tween 60 and NaCl on the corrosion of cold rolled steel in 0.5M sulfuric acid, Corros. Sci. 47: 1932–1952. |
| [18] | Nnanna, L.A., Onwuagba, B.N., Mejeha, I.M., Okeoma, K.B. 2010, Inhibition effects of some plant extracts on the acid corrosion of aluminium alloy, Afr. J. Pure Appl. Chem. 4(1): 11-16. |
| [19] | Nnanna, L.A. Uchendu, K.O. Nwosu, F.O., Ihekoronye, U., Eti P.E. 2014, Gmelina arborea bark extracts as corrosion inhibitor for mild steel in an acidic environment, Int. J. Mater. Chem., 4(2): 34-39. |
| [20] | Noor, E.A. 2007, Temperature effects on the corrosion inhibition of mild steel in Acidic solutions by aqeous extract of Fenugreek leaves, Int. J. Electrochem. Sci. 2: 996-1017. |
| [21] | Nwokocha, L.M., Ogunmola, G.B. 2005, Isolation and characterization of starch from Treculia africana, Decne (African breadfruit) seeds, Niger. J. Sci. 39: 73–79. |
| [22] | Obot, I.B., Obi-Egbedi, N.O., Umoren, S.A. 2009, Adsorption characteristics and corrosion inhibitive properties of clotrimazole for aluminium corrosion in hydrochloric acid, Int. J. Electrochem. Sci. 4: 863-877. |
| [23] | Odiongenyi, A.D., Odoemelam, S.A., Eddy, N.O. 2009, Corrosion inhibition and adsorption properties of ethanol extract of Vernonia Amygdalina for the corrosion of mild steel in H2S04, Portugaliae Electrochimica Acta, 27(1): 33-45. |
| [24] | Oguzie, E.E., Unaegbu, C., Ogukwe, C.N., Okolie, B.N., Onuchukwu, A.I. 2004, Inhibition of mild steel corrosion in sulphuric acid using indigo dye and synergistic halide additives, Mater. Chem. Phy., 84: 363-368. |
| [25] | Oguzie, E.E. 2007, Corrosion inhibition of aluminum in acidic and alkaline media by sansevieria trifasciata extract. Corros. Sci. 49: 1527-1539. |
| [26] | Oguzie, E.E. 2005, Inhibition of acid corrosion if mild steel by Telfaria occidentalis extract. Pig. Resin Technol. 34(6): 321-326. |
| [27] | Oguzie, E.E. 2008, Evaluation of the inhibitive effect some plant extracts on the acid corrosion of mild steel. Corros. Sci. 50: 2993-2998. |
| [28] | Oguzie,E.E 2006, Adsorption and corrosion inhibitive properties of Azadicachta indica in acid solutions. Pig. Resins Technol, 35(6): 334-340. |
| [29] | Okafor, P.C., Ekpe, U.J., Ebenso, E.E., Umoren, E.M., Leizou, K.E. 2005, Inhibition of mild Steel corrosion in acidic medium by Allium Sativum extracts. Bull. Electrochem. 21(8): 347-352. |
| [30] | Okafor, P.C., Ikpi, M.E., Uwah, I.E., Ebenso, E.E., Ekpe, U.J., Umoren, S.A. 2008, Inhibitory action of phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros. Sci. 50, 2310-2317. |
| [31] | Patel, N.S., Jauhari, S., Mehta, G.N. 2009, Mild steel corrosion inhibition by Bauchinia Purpurea leaves extract in 1N sulphuric acid. Arab. J. Sci Eng., 34(2c): 61-69. |
| [32] | Sethuraman, M.G., Botha Raja, P. 2008, Corrosion inhibition of mild steel by Datura metel in acidic medium, Pig. Resin Technol. 34(6): 327-331. |
| [33] | Sharmila A., Prema, A., Sahayaraj, A. 2010, Influence of Murraya koenighii extract on the corrosion inhibition of carbon steel in HCl solution, Rasayan J. Chem. Sci. 3(1): 74-81. |
| [34] | Subha. R., Saratha, R. 1999, Naturally occurring substance (Calendula Officialis flower) as a corrosion inhibitor for mild steel in 1M HCl solution. Ph.D Thesis. Department of Chemistry, Avinashilingam Deemed University Coinbatore. |
| [35] | Umoren S.A., Obot I.B., Ebenso, E.E., Obi-Egbedi, N. 2008, Studies on the inhibitive effect of exudate gum from Dacroydes edulis on the acid corrosion of aluminium, Portugaliae Electrochimica Acta, 26: 199-209. |
| [36] | Umoren, S.A. 2008, Inhibition of aluminium and mild steel corrosion in acidic medium using gum Arabic, Cellulose, 15: 751-761. |
| [37] | Zhumdahl, S.S. 1993, Chemistry 3rd Edn. D.C. heath and co. Lexington. Toronto. Canada pp 834-85. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML