-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2015; 5(1): 4-13
doi:10.5923/j.ijmc.20150501.02
Response Surface Optimization of the Inhibition Efficiency of Gongronema latifolium as an Inhibitor for Aluminum Corrosion in HCl Solutions
Chigoziri N. Njoku, Okechukwu E. Onyelucheya
Department of Chemical Engineering, Federal University of Technology, Owerri, Nigeria
Correspondence to: Okechukwu E. Onyelucheya, Department of Chemical Engineering, Federal University of Technology, Owerri, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The inhibiting effects of Gongronemalatifolium on corrosion of aluminum in 0.5M and 2M HCl solution was investigated using gravimetric technique at temperatures of 303K and 333K. The results indicated that the extracts inhibited the corrosion process in the acid media and inhibition efficiency improved with inhibitor concentration but decreased with increase in temperature which suggests a physical adsorption. The analysis of the results showed that the linear and quadratic effects of the process variables were highly significant, as well as, the interaction effect of temperature and time. The optimum conditions obtained were, Acid Concentration 1.73mol/l; Temperature 328.62 K; Time 10.14 hours; Extract Concentration 1000mg/L; and the Optimum Inhibition Efficiency at this optimum condition was predicted to be 74.14%. Gravimetric experiments were carried out at these optimum conditions to validate the predicted optimum values. The obtained experimental value of 75.43% agreed closely with that obtained from the regression model. This study has revealed that Gongronemalatifolium (GL) extract is a good inhibitor for the corrosion of aluminum in HCl solution and the performance can be improved by carefully moderating the process parameters.
Keywords: Corrosion inhibition, Inhibition efficiency, Response surface optimization, Gongronemalatifolium
Cite this paper: Chigoziri N. Njoku, Okechukwu E. Onyelucheya, Response Surface Optimization of the Inhibition Efficiency of Gongronema latifolium as an Inhibitor for Aluminum Corrosion in HCl Solutions, International Journal of Materials and Chemistry, Vol. 5 No. 1, 2015, pp. 4-13. doi: 10.5923/j.ijmc.20150501.02.
Article Outline
1. Introduction
- Aluminum is a hard, strong, white metal. It has a density around one third that of steel or copper making it one of the lightest commercially available metals. The resultant high strength to weight ratio makes it an important structural material. The addition of alloying elements like Manganese, Silicon, Copper and Magnesium can increase the strength properties of Aluminium and produce an alloy with properties tailored to particular applications. It has the advantage over steel in that its tensile strength increases with decreasing temperature while retaining its toughness. It is highly electropositive and resistant to corrosion because a hard, tough film of oxide is formed on the surface [1]. The surface film is amphoteric; hence the metal could dissolve readily in both strong acid and alkaline media. Hydrochloric acid solutions are normally used for pickling of aluminum and electrochemical etching processes that normally lead to substantial loss of metal to corrosion. Aluminum is extensively used in industry as well as domestic applications. Corrosion of metals is a major industrial problem that has attracted much investigation and researches [2-4]. Corrosion is the primary means by which metals deteriorate. Most metals corrode on contact with water (and moisture in the air), acids, bases, salts, oils, aggressive metal polishes, and other solid and liquid chemicals. The corrosion of aluminum in acidic solution is the most common form of corrosion and has practical importance during industrial processes such as acid pickling of iron and steel, chemical cleaning of scale in metallurgy, oil recovery in the petroleum industries, etc. [5-8]. Because of the general aggression of acid solutions, inhibitors are commonly used to reduce the corrosive attack on metallic materials. Inhibitors allow the reduction of corrosion rate by influencing the kinetics of corrosion processes which are consistent with anodic and cathodic conjugate reactions. Corrosion behavior of aluminum has been studied in acid media. It has been reported that addition of halide salt to sulphuric acid solution containing organic inhibitor, effectively inhibits iron corrosion [2, 9]. Halides have been reported to inhibit corrosion of some metals in strong acids and this effect depends on ionic size and charge. The use of chemical inhibitors has been a concern about its toxicity which affects the living organism as well as poisoning of environment. The use of plant extracts to prevent corrosion has become important because they are environmentally acceptable, readily available and renewable source for a wide range of inhibitors. Plant extracts are rich source of naturally synthesized chemical compounds that can be extracted by simple methods with low cost [10]. These extracts are biodegradable and do not contain heavy metals or other toxic compounds. The use of plant extracts as corrosion inhibitor of metals in acidic and alkaline media have been reported [11-15]. The present work focuses on the Response Surface Optimization of the inhibition efficiency of GL as an inhibitor for Aluminum Corrosion in HCl Solutions. The effects of the process parameters namely, the Extract Concentration, Temperature, Time and Acid Concentration on the corrosion process were studied using a Central Composite Design (CCD). This method, and indeed other methods of Design of Experiment, studies the linear, square and interaction effects of the process factors thereby providing the best approach for establishing a model correlating the response variable and the independent variables affecting the inhibition efficiency. The traditional one factor at a time (OFAT) method of analysis does not take into account the interaction effect between factors and involves a greater number of experimental runs.The approach of the present study involves the following steps;(a) Design of the experiments using the CCD to obtain the points where the experimental runs will be performed.(b) Experimental observation of the corrosion inhibition effects of the various factors at the design points.(c) Obtaining a mathematical model expressing the relationship between the process factors and the percentage Inhibition Efficiency which is the system response.(d) Prediction of the optimum values of the process parameters for maximum Corrosion Inhibition Efficiency using Response Surface methodology.(e) Experimental verification of the conditions predicted by the model.
2. Materials and Methods
2.1. Material Preparation
- Aluminum sheets of AAI060 and purity 98.98% were obtained from Material and Metallurgical Engineering Workshop, Federal University of Technology, Owerri, Nigeria. The composition of AA1060 Aluminum alloy is as follows: Si – 0.25%, Fe – 0.35%, Cu – 0.05%, Mn – 0.03%, Mg – 0.03%, Zn – 0.05%, Ti – 0.03%, V – 0.05%, Tensile Strength – 83 MPa, Hardness (HB500) – 23, Shear Strength – 55MPa. Each sheet was 0.1 cm in thickness and mechanically press-cut into coupons of dimension 3cm x 3 cm. The coupons were descaled using wire brush and degreased in absolute ethanol, dried in acetone, weighed and stored in moisture- free desiccator prior to use.
2.2. Extraction of Plant
- The solvents used for extraction of Gongronema latifolium leaf is ethanol. All the solvents used in this study were of analytical reagent grade. Sample leaves of Gongronema latifolium (Utazi) were obtained from Afor Ihitta ogada market in Owerri North, Imo State, Nigeria. The leaf samples were washed and dried under the sunlight and ground to fine powder. 20 g of leaf extract were extracted with Soxhlet extractor for 3 hours using 800 mg/L of ethanol. After extraction the samples were cooled and filtered. The filtrates obtained were used to prepare inhibitor concentrations in HCl corrodents.
2.3. Phytochemical Test
- The extract was subjected to phytochemical screening to detect the presence of Secondary metabolites such as alkaloids, flavonoids, tannins and steroids. The leaves of Gongronema latifolium were separated, washed with water, sun dried and powered for extraction, a known amount of powder (20g) was subjected each to Soxhlet extraction and exhaustively extracted with ethanol for 12hours. The extract was filtered, concentrated in vacuum under reduced pressure using rotary flash evaporator and dried. The extract was subjected to phytochemical screening to detect the presence of secondary metabolites.
2.3.1. Quantitative and Qualitative Analysis
2.3.1.1. Determination of Alkaloids
- This was done by the alkaline precipitation gravimetric method [16]. A measured weight of the sample was dispersed in 10% acetic acid solution in ethanol to form a ratio of 1:10 (10%). The mixture was allowed to stand for 4hours at 28℃. It was later filtered via Whatmann No 42 grade of filter paper. The filtrate was concentrated to 1/4 of its original volume by evaporation and treated with drop wise addition of concentrated aqueous NH4OH until the alkaloid was precipitated. The alkaloid precipitated was received in a weighed filter paper, washed with 1% ammonia solution dried in the oven at 80℃. Alkaloid content was calculated and expressed as a percentage of the weight of sample analyzed.
2.3.1.2. Determination of Flavonoids
- 5g of the sample was boiled in 50ml of 2M HCl solution for 30mins under reflux [16]. It was allowed to cool and then filtered through Whatmann No 42 filter paper. A measured volume of the extract was treated with equal volume of Ethyl Acetate. The flavonoid precipitated was recovered by filtration using weighed filter paper. The resulting weight difference gave the weight of flavonoid in the sample.
2.3.1.3. Determination of Tannin
- Tannin content was determined by Folis-Denis colorimetric method [16]. 5g sample was dispersed in 50mls of distilled water and shaken. The mixture was allowed to stand for 30min at 28℃ before it was filtered through Whatmann No. 42 grade of filter paper. 2mls of the extract was dispersed into 50ml volumetric flask. Similarly 2ml standard tannin solution (tannic acid) and 2ml of distilled water were put in separate volumetric flasks to serve as standard and reagent was added to each of the flask and the 5ml of saturated Na2CO3 solution added. The content of each flask was made up to 50mls with distilled water and allowed for 90min. Their respective absorbance was measured in a spectrophotometer at 260nm using the reagent blank to calibrate the instrument at zero.
2.3.1.4. Determination of Steroids
- This was determined by the method described by [16]. 5g of each sample was dispersed in 100ml freshly distilled water and homogenized in a laboratory blender. The homogenate were filtered and the filtrate was eluted with normal ammonium hydroxide solution (pH 9). 2ml of elute were put in test tube and mixed with 2ml of chloroform. 3ml of ice-cold acetic anhydride were added to the mixture in the flask and 2 drops of conc. H2SO4 were cautiously added to cool. Standard sterol solution was prepared and treated as described above. The absorbance of the standard and prepared sample were measured in a spectrometer at 420nm.
2.4. Gravimetric Method
- In weight loss experiment, a hole with diameter 0.5 cm was drilled in each aluminum coupon so that it could hang freely in solution. The aluminum coupons were suspended in beakers containing 200 ml of test solution maintained at 303 and 333 K with glass hooks and rods in a thermo-static water bath. The test solutions used were 0.5M and 2.0M HCl solutions. The weight loss was determined by retrieving coupons from test solutions at 3 hours intervals for 12 hours. The coupons after being retrieved from test solution were scrubbed with bristle brush under running water, dried in acetone and weighed [17]. The difference in weight was taken as weight loss of aluminum. From weight loss, the Inhibition Efficiency (%I.E) of extract and Corrosion Rate (CR) of aluminum were calculated using equations 1 and 2, respectively.
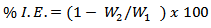 | (1) |
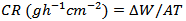 | (2) |
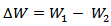 The degree of surface coverage was calculated using:
The degree of surface coverage was calculated using: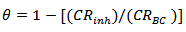 | (3) |
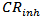 is corrosion rate for aluminum in presence of inhibitor, and
is corrosion rate for aluminum in presence of inhibitor, and 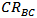 is corrosion rate for aluminum in the absence of inhibitor.
is corrosion rate for aluminum in the absence of inhibitor.2.5. Design of Experiment
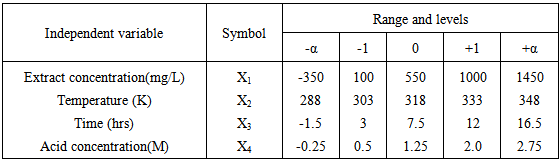
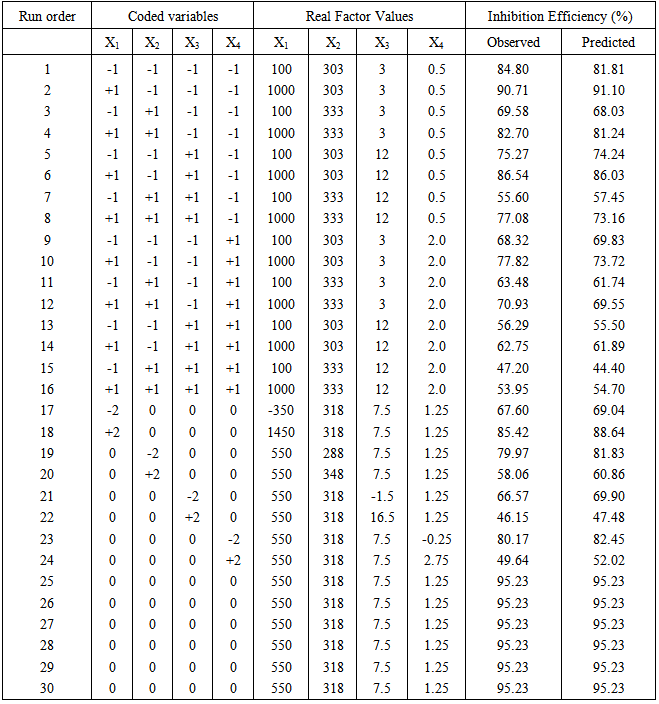
- In order to examine the combined effect of the four different factors (extract inhibitor concentration, acid concentration, exposure time and temperature) on percentage inhibition efficiency (% I.E) and derive a model, a Central Composite Factorial Design of 24 = 16, plus 6 centre points and (2 x 4 = 8) star points leading to a total of 30 experiments were performed on the plant extract. The factor levels with the corresponding real values are shown in Table 1, while the design matrix is shown in table 2. The matrix for the four variables was varied at five levels (-α, -1, 0, +1, and +α). As usual, the experiments were performed in random order to avoid systematic error.
|
|
2.6. Statistical Analysis
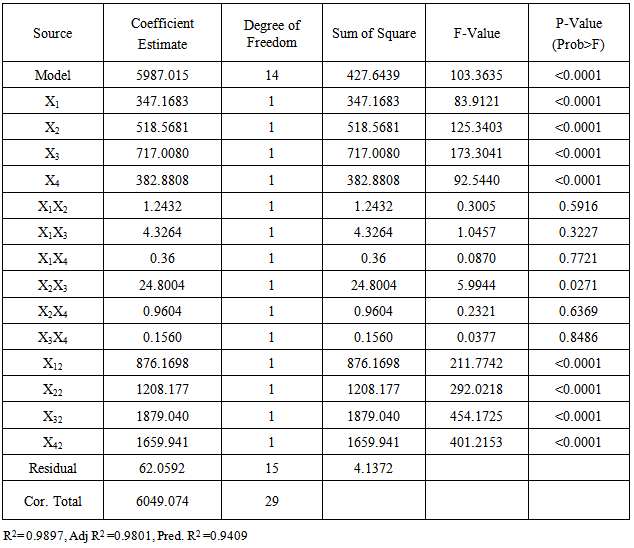
- The generated experimental data were analyzed using the Design Expert 8.0.7.1 software by StatSoft Inc. USA to obtain the Analysis of Variance (ANOVA), Regression Analysis, and Response Surface plots of the interaction effects of the factors to evaluate optimum conditions for the Corrosion Inhibition process. The linear, quadratic, and linear interactive effects of the process variables on the inhibition efficiency were calculated and their respective significance evaluated by ANOVA test. The p-value was used as the yardstick for measuring the significance of the regression coefficients, values of p less than 0.05 signified that the coefficient is significant. The experimental data were fitted to the Second-Order polynomial regression model and the adequacy of the model tested by the coefficient of determination (R2) value as compared to the adjusted R2 value.
3. Results and Discussions
3.1. Phytochemical Test
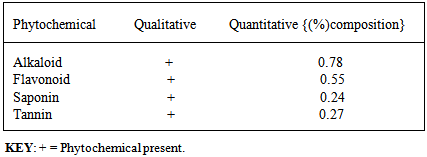
- The objective of this analysis was to determine the content of phytochemicals present in the extract. The qualitative analysis tests were carried out to ascertain the presence of the different phytochemicals in the plant before conducting the quantitative analysis which gave insight to the proportions. The phytochemical composition of the ethanol extract of GL shows it contains alkaloids, flavonoids, saponins, and tannins (table 3). This indicates that the inhibition efficiency of the extract is due to the presence of some or all of the above listed phytochemical constituents [15]. [18] also stated that saponins, tannins and alkaloids are active constituents of most green inhibitors.
|
3.2. Evaluation of Regression Model for Inhibition Efficiency (%I.E)
- The correlation between the experimental process variables and the inhibition efficiency was evaluated using the central composite modeling technique. A polynomial quadratic regression equation of the form Y=b0+b1X1+b2X2+b3X3+b4X4+b5X1X2+b6X1X3+b7X1X4+b8X2X3+b9X2X4+b10X3X4+b11X12+b12X22+b13X32 +b14X42 were fitted between the response (% Inhibition Efficiency, (Y)) and the process variables: extract concentration (X1), temperature (X2), time (X3), and Acid concentration(X4). The model in terms of the coded values of the process parameters is given by:
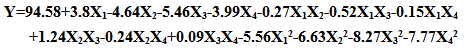 | (4) |
|
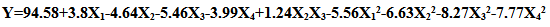 | (5) |
3.3. Surface Response Plots for Inhibition on Aluminum
- The interactive effects of the process variables on the percentage inhibition efficiency were studied by plotting three dimensional surface curves against any two independent variables, while keeping other variables at their central (0) level. The 3D curves of the response (% inhibition efficiency) and contour plots from the interactions between the variables are shown in figures 2 to 7, for GL on aluminum. From the interactive effects of extract concentration and temperature in figure 2, we can observe that inhibition efficiency of aluminum decreases with an increase in temperature but increases with an increase in the concentration of the extract inhibitor of GL. The phytochemcial constituents of plant extract, such as tannin, saponnin, alkaloids, glycoside, and steroids are the major factors that determine the inhibition efficiency of the plant extract [19]. In figure 3, inhibition efficiency of aluminum decreases with an increase in time, also an increase in acid concentration, inhibition efficiency is decreased, as shown in figure 4, but increases with an increase in the extract concentration. Similarly, the interactive effects of temperature and time as shown in figure 5, inhibition efficiency decreased with increase in both temperature and time, this could be attributed to the fact that the longer the metal stay in the acid the more it corrodes and at a higher temperature, the metal becomes physically adsorbed [19]. Similarly, inhibition efficiency decreased with increase in acid concentration as shown in figures 6 and 7. The optimum conditions were predicted with the Design Expert software with; acid concentration 1.73mol/l; temperature 328.62 K; time 10.14 hours; extract concentration 1000mg/L; and the optimum inhibition efficiency at this optimum condition were predicted to be 74.14%. Experiments were carried out at these optimum conditions to validate the predicted optimum values. The experimental value of 75.43% agreed closely with that obtained from the regression model.
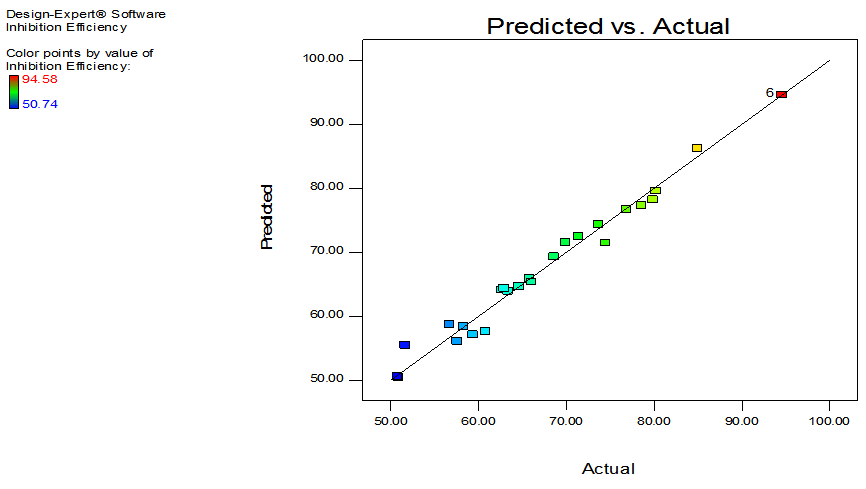 | Figure 1. Plot of predicted values versus the actual experimental values for inhibition of GL on aluminum |
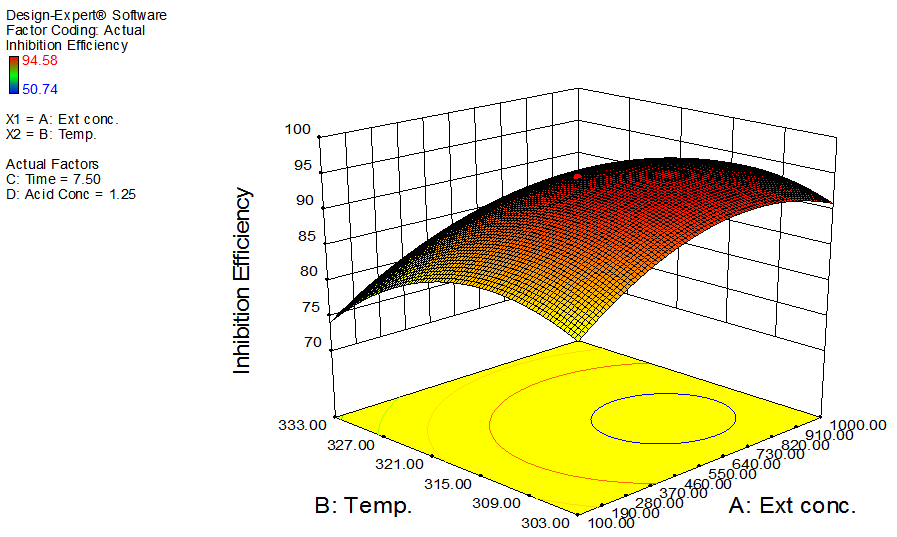 | Figure 2. Effects of extract concentration and temperature on inhibition efficiency of GL on aluminum at constant time and acid concentration |
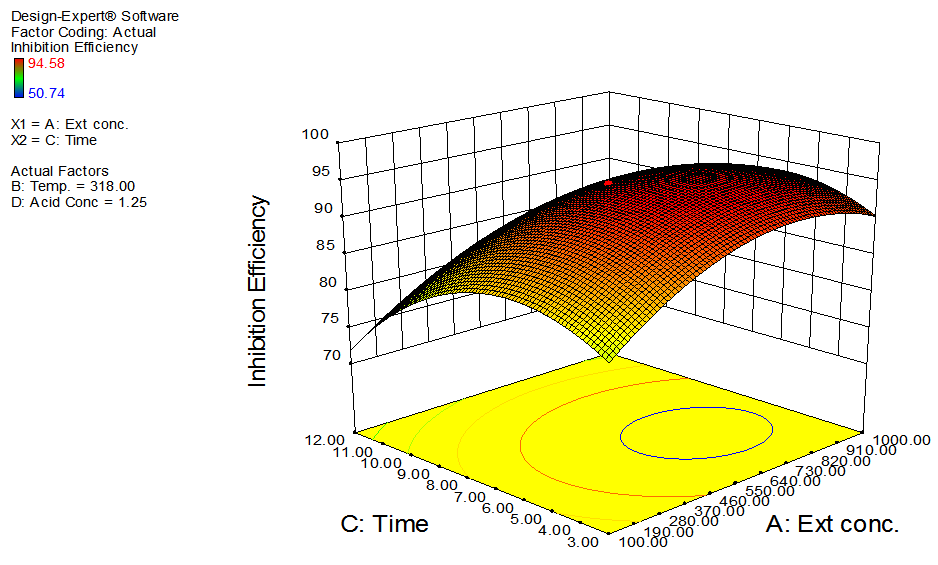 | Figure 3. Effects of extract concentration and time on inhibition efficiency of GL on aluminum at constant temperature and acid concentration |
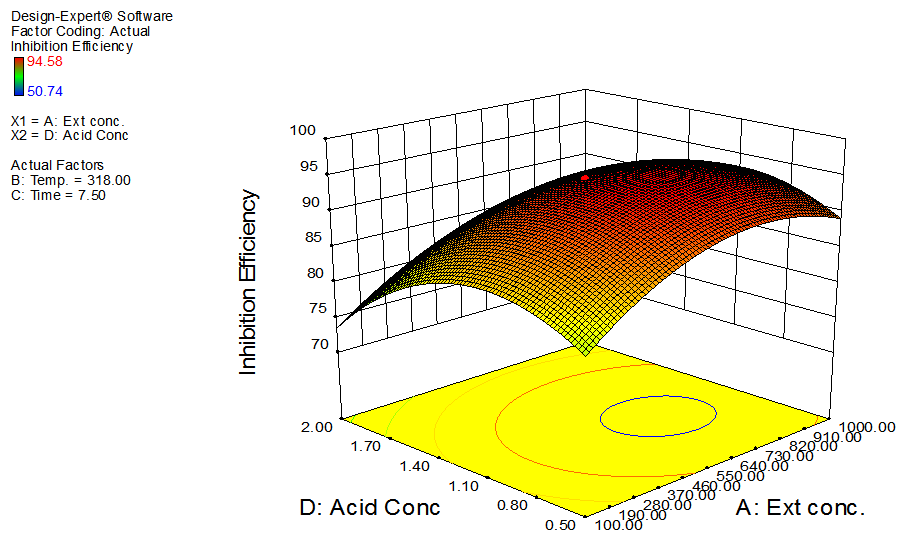 | Figure 4. Effects of extract concentration and acid concentration on inhibition efficiency of GL on aluminum at constant temperature and time |
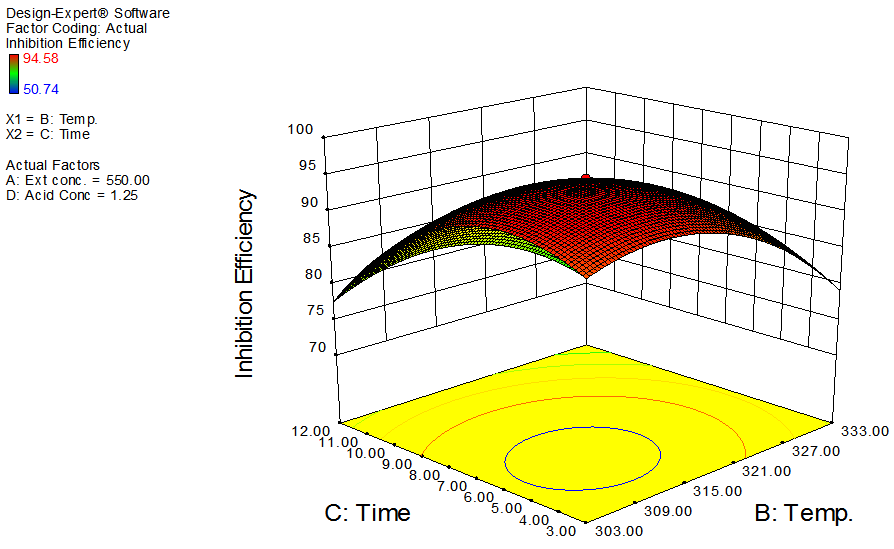 | Figure 5. Effects of temperature and time on inhibition efficiency of GL on aluminum at constant extract concentration and acid concentration |
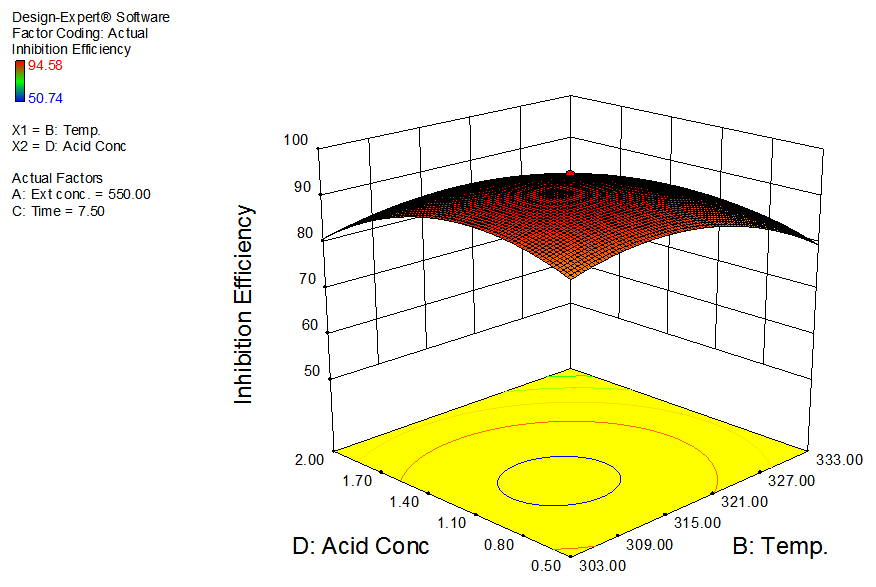 | Figure 6. Effects of temperature and acid concentration on inhibition efficiency of GL on aluminum at constant extract concentration and time |
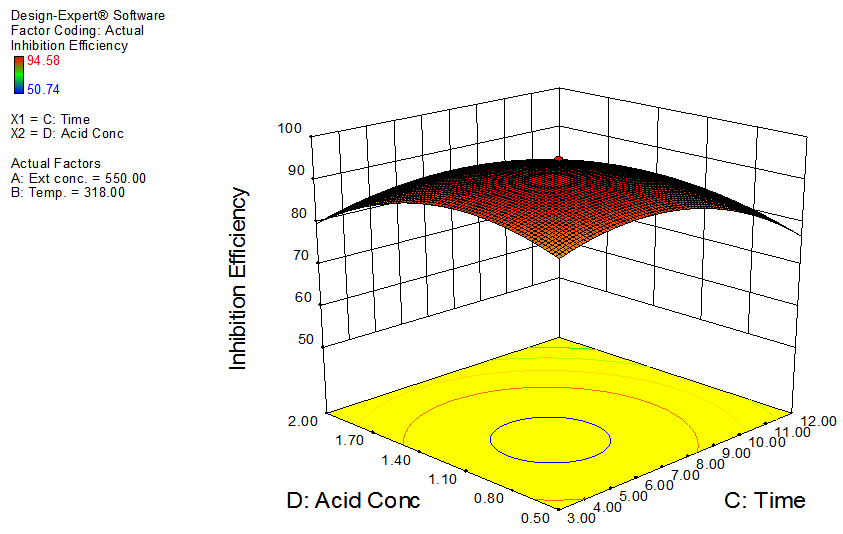 | Figure 7. Effects of time and acid concentration on inhibition efficiency of GL on aluminum at constant extract concentration and temperature |
4. Conclusions
- The central composite design and response surface methodology enabled the determination of optimal operating conditions for percentage inhibition efficiency. The validity of the model was proven by fitting the values of the variables to the model equation and by carrying out experiments using these values. The optimization of the analyzed responses demonstrated that the best results were acid concentration 1.73mol/l; temperature 328.62 K; time 10.14 hours; extract concentration 1000mg/L; and the optimum inhibition efficiency at this optimum condition was predicted to be 74.14%. All points were located near the central point of the design. The process parameters investigated were all significant both at the linear and quadratic level and only the interaction effect of temperature and reaction time was statistically significant. Experiments were carried out at these optimum conditions to validate the predicted optimum values. The experimental value of 75.43% agreed closely with that obtained from the regression model.
ACKNOWLEDGEMENTS
- Oguzie, E.E. (Electrochemistry and Materials science Research Unit, (EMRU) Department of Chemistry, Federal University of Technolology, Owerri Nigeria) is acknowledged for providing facilities for Gravimetric, Characterization and Phytochemical analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML