-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2015; 5(1): 1-3
doi:10.5923/j.ijmc.20150501.01
Study of Thermal Stability of Nitrile Rubber/Polyimide Compounds
Mohaimen Alneamah, Mohammed Almaamori
Department of polymer engineering, college of materials engineering, University of Babylon, Babylon, Iraq
Correspondence to: Mohaimen Alneamah, Department of polymer engineering, college of materials engineering, University of Babylon, Babylon, Iraq.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Nitrile rubber compounds were prepared by the addition of polyimide (PI). The effect of polyimide on thermal stability of nitrile rubber was studied. Both thermogravimetric analysis (TGA) and dynamic mechanical analysis (DMTA) of the prepared compounds showed much better thermal stability. The onset temperature of nitrile rubber increased from 360℃ to 368.4℃. Also glass transition temperature of nitrile rubber increased from -5℃ to 11.6℃. This proved that the presence of polyimide in nitrile rubber matrix can increase thermal stability.
Keywords: Nitrile rubber, Polyimide, Thermal stability
Cite this paper: Mohaimen Alneamah, Mohammed Almaamori, Study of Thermal Stability of Nitrile Rubber/Polyimide Compounds, International Journal of Materials and Chemistry, Vol. 5 No. 1, 2015, pp. 1-3. doi: 10.5923/j.ijmc.20150501.01.
Article Outline
1. Introduction
- The measure of thermal stablity of a material is the degree of change in thermal properties with temperature. This change is caused by chemical (bonds scission, oxidation, etc.) or physical changes in polymer structure resulting in a material with different properties (usually worse). The study of thermal stability of polymers and how they react with high temperatures is very important in the design of new and better polymer products.Nitrile rubber compounds show a limited high temperature stability. Many researches have been made to enhance thermal stability of nitrile rubber either by blending with another rubber [1] or other polymers [2, 3] or by using different additions such as hybrid filler systems [4, 5]. Allows the addition of different kinds of fillers and study its effect on thermal stability of nitrile rubber [6-10]The aim of the work presented here was to determine the effect of high temperature polymer (polyimide) on thermal stability of nitrile rubber.
2. Experimental
2.1. Materials
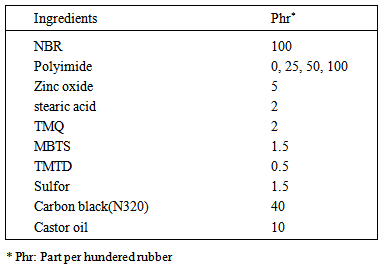
- Nitrile butadiene rubber (ACN content 45%), zinc oxide, stearic acid, tetramethylthiuram disulfide (TMTD), mercaptobenzothiazole disulfide (MBTS), castor oil, Sulfor, 2,2,4-trimethyl-1,2-dihydroquinoline (TMQ) and Carbon black (N 326) were supplied by Babylon tires company. Polyimide (aliphatic) was supplied by Taizhou Huangyan Donghai Chemical CO.
2.2. Preparation
- Nitrile rubber compounds were mixed by two roll mill according to ASTM D 3182. Table 1 shows the formulation of the prepared NBR compounds. Vulcanization was done in 150℃ for 15 min.
|
2.3. Characterization
2.3.1. Thermogravimetric Analysis
- Thermograms were obtained by means of a thermogravimetric analyzer (TGA/SDTA 851e Mettler Toledo). The measurements were made under a heating rate of 20 ℃/min at a temperature range 25℃ to 600 ℃. The experiments were done in atmosphere. About 15–16 mg of the sample was used for the analysis.
2.3.2. Dynamic Mechanical Analysis
- The dynamic loss factor (tanδ) was measured as a function of temperature with a dynamic mechanical thermal analyzer (METTLER TOLEDO dynamic mechanical thermal analyzer was used) under the tension mode at a frequency of 1 Hz. The strain amplitude in the temperature range of (-50 to 280℃) was 0.1%. The heating rate was 5℃/min.
3. Results and Discussions
3.1. Thermogravimetric Analysis
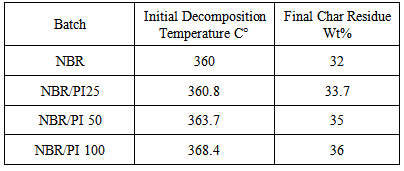
- Thermogravimetric analysis (TGA) curves for the prepared compounds are shown in figure 1. Nitrile rubber compounds present only one thermal degradation step in the temperature range of 25℃-600℃. The incorporation of polyimide in to NBR causes shift towards higher temperatures. Table 2 shows the initial decomposition temperature at weight loss of 5% of NBR compounds. Initial decomposition temperature increased from 360℃ for NBR compound without polyimide to 368.4℃ for NBR compound with 100 (Phr) polyimide. Final char residue of the prepared compounds increased from 32 wt% to 36 wt% for NBR with 100 (Phr) polyimide table 2. All this proves that the incorporation of polyimide in to NBR matrix increases thermal stability of NBR.
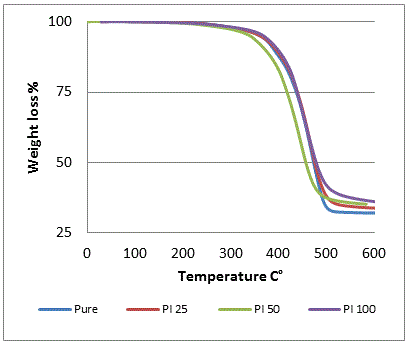 | Figure 1. TGA curves of nitrile rubber compounds |
|
3.2. Dynamic Mechanical Analysis
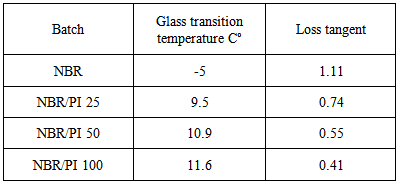
- Curves of Dynamic mechanical analysis (DMA) of the prepared compounds are shown in figure 2. Table 3 summarizes the effect of polyimide addition on glass transition temperature (Tg) and loss tangent (tanδ) of nitrile rubber. Tg of NBR increased from -5℃ to 11.6℃.
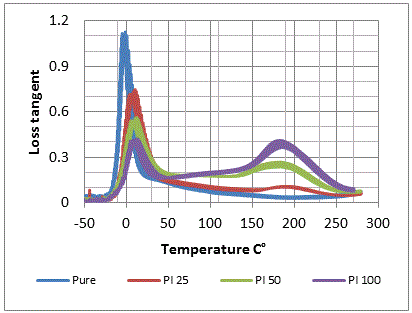 | Figure 2. DMTA curves of nitrile rubber compounds |
|
4. Conclusions
- NBR compounds were prepared by the addition of polyimide (PI) as a high temperature filler. Thermal stability of NBR compounds was studied. It was found that the initial decomposition temperature at 5% weight loss for NBR compound without polyimide increase as polyimide content increase also high Tg values of the prepared compounds which increase with polyimide content increase are a clear evidence of improving thermal stability of nitrile rubber by the addition of polyimide.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML