-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2014; 4(4): 92-99
doi:10.5923/j.ijmc.20140404.03
Mass Spectral Fragmentation Modes of Some New Pyrimidinethiones, Thiazolo[3,2-a]Pyrimidines and Bis-Pyrimidines
Mounir A. I. Salem1, Tarik E. Ali2, Magda I. Marzouk1, Marwa S. Salem1, Ghazala A. Al-Shibani1
1Department of Chemistry, Faculty of Science, Ain Shams University, Cairo, Egypt
2Department of Chemistry, Faculty of Education, Ain Shams University, Cairo, Egypt
Correspondence to: Tarik E. Ali, Department of Chemistry, Faculty of Education, Ain Shams University, Cairo, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Mass fragmentation pathways of a number of pyrimidinethiones, thiazolo[3,2-a] pyrimidines and bis-pyrimidines were investigated by electron impact mass spectrometry (EI-MS). The molecular ion peaks M+2 and M+ were recorded at different intensities. Characteristic fragment ions were formed by successive loss of simple functional groups followed by decomposition of the heterocycles connected to pyrimidine ring.
Keywords: Mass spectrometry, Fragmentation processes, Pyrimidines
Cite this paper: Mounir A. I. Salem, Tarik E. Ali, Magda I. Marzouk, Marwa S. Salem, Ghazala A. Al-Shibani, Mass Spectral Fragmentation Modes of Some New Pyrimidinethiones, Thiazolo[3,2-a]Pyrimidines and Bis-Pyrimidines, International Journal of Materials and Chemistry, Vol. 4 No. 4, 2014, pp. 92-99. doi: 10.5923/j.ijmc.20140404.03.
Article Outline
1. Introduction
- The pyrimidine ring is the building unit of DNA and RNA which explains the fact that pyrimidine derivatives exhibit diverse pharmacological activities, the most pronounced of which are anticancer, [1, 2] antiviral, [3, 4] anti-HIV, [5, 6] antiamoebic activity [3] and antimicrobial activities. [7] They also showed activity against gonadotropin releasing hormone receptors [8] as well herbicidal activity targeting acetohydroxyacid synthase, which catalyzed the first common step in branched-chain amino acid biosynthesis. [9] Mass spectrometry was applied to the characterization of the compounds using analysis of metastable ions by the collision induced dissociation technique and exact mass measurements. [10-14] However, to best our knowledge, there are some few mass spectral fragmentation studies for isolated and fused pyrimidines. [15-17] In continuation of our work on the mass fragmentation mechanisms of the heterocyclic compounds, [18-21] a detailed study on a number of pyrimidinethiones, thiazolopyrimidines and bis-pyrimidines, was performed to understand the fragmentation modes of these compounds. Thus, this paper reports a study on the fragmentation mechanisms under electron ionization conditions of some new pyrimidinethiones, thiazolopyrimidines and bis-pyrimidines. It has been possible to make some generalizations regarding fragmentation modes of their molecular ions.
2. Experimental
2.1. Synthesis of the Studied Compounds
- The studied compounds 1-8 were synthesized as shown in Schemes 1 and 2. Details of the synthetic methods are reported in our recent article. [22] Also, all the compounds were previously characterized by elemental analysis, MS, IR, 1H, and 13C-NMR spectra [22].
2.2. MS Measurements
- The electron ionization mass spectra were recorded on Shimadzu GCMS-QP-1000EX mass spectrometers at 70 eV in Microanalytical center at Cairo University, Giza, Egypt. The electron ionization ion source was kept at 200ºC. The compounds were introduced with a probe which was ballistically heated to 250ºC. The EI mass spectra were obtained over the range of m/z 50-650.
3. Results and Discussion
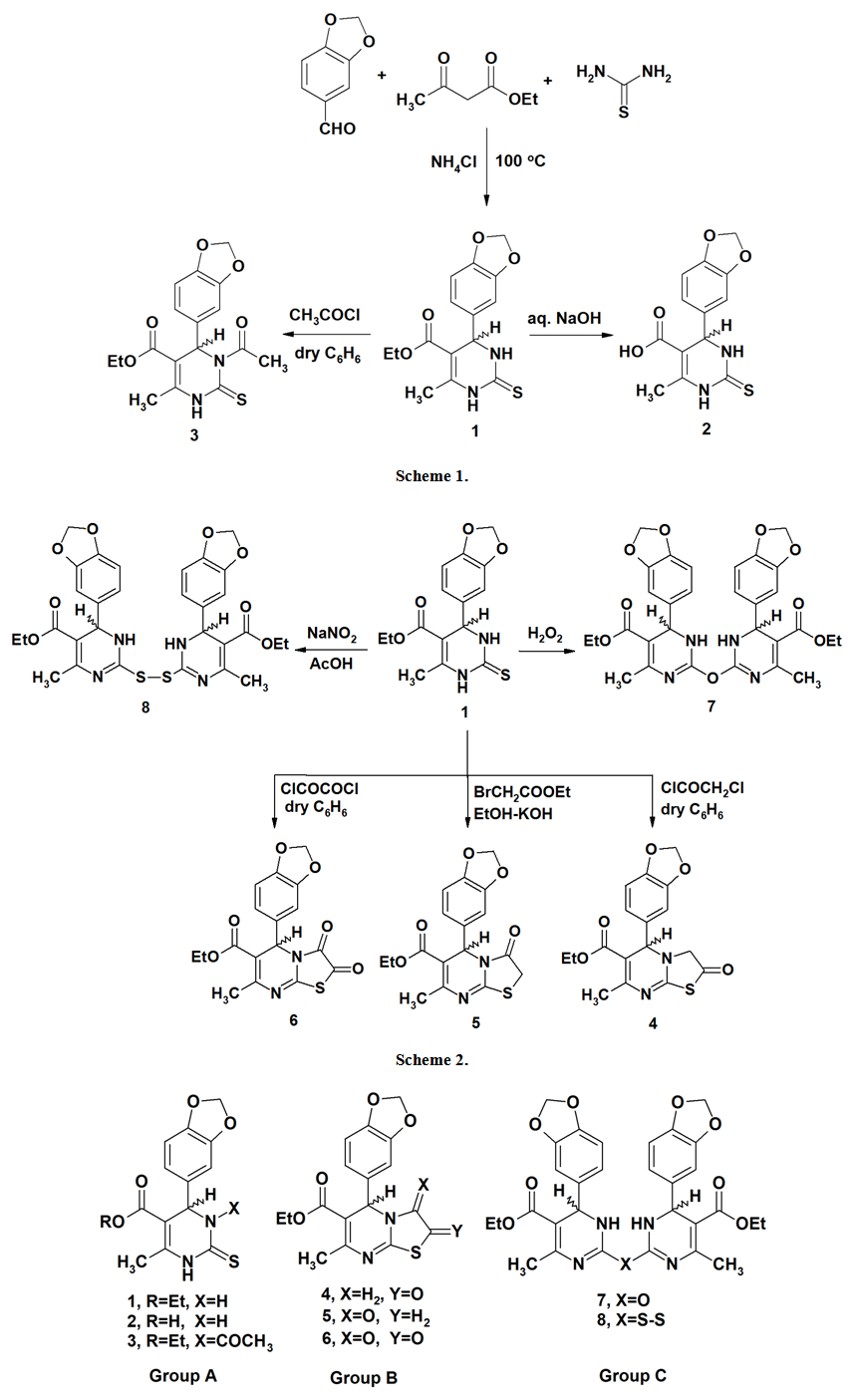
- For a better understanding of mass fragmentation routes, the compounds under study were divided into three groups according to their structural patterns: A (pyrimidinethiones), B (thiazolopyrimidines) and C (bis-pyrimidines) (Figure 1).
 | Figure 1. |
 | Figure 2. |
 | Figure 3. |
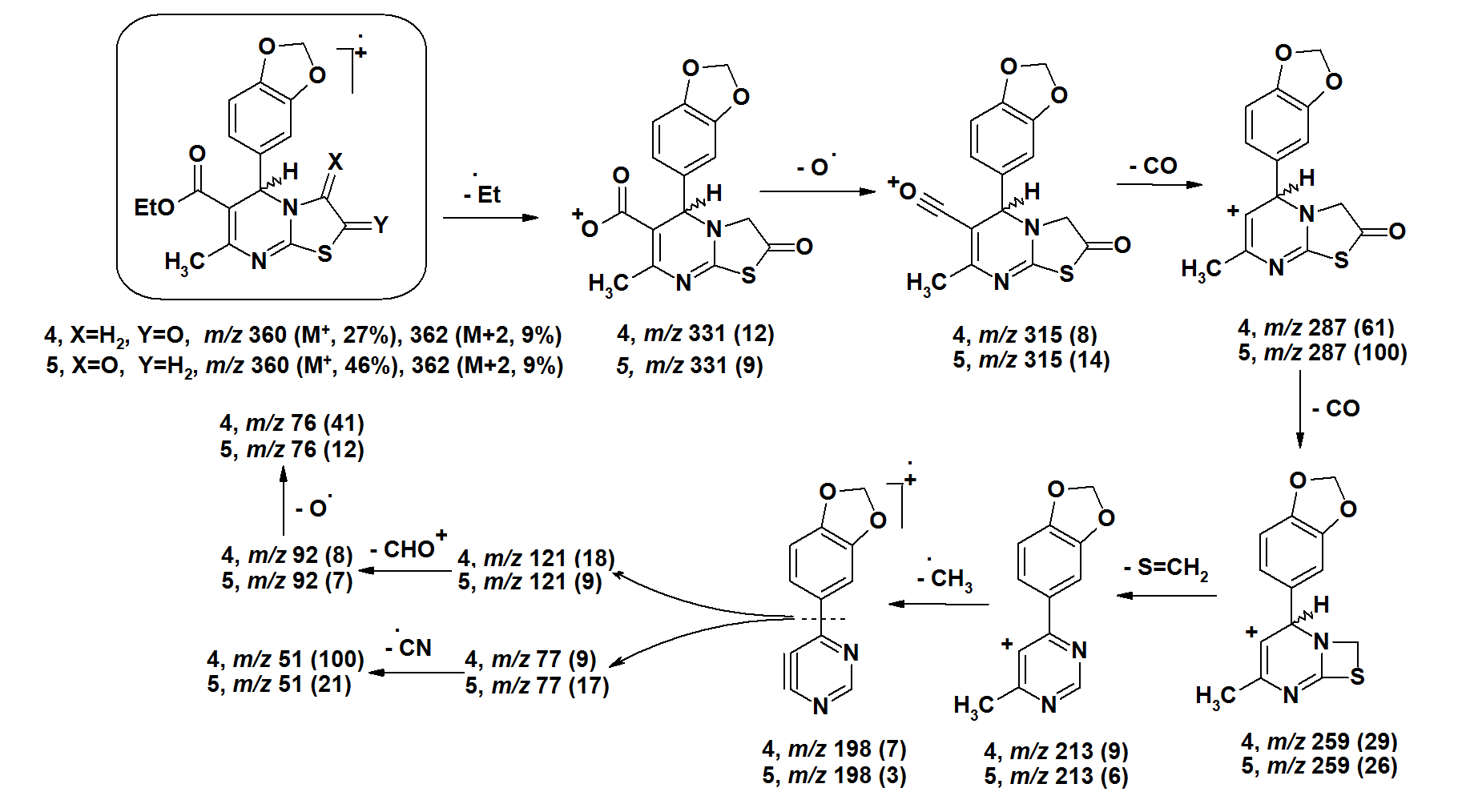 | Figure 4. |
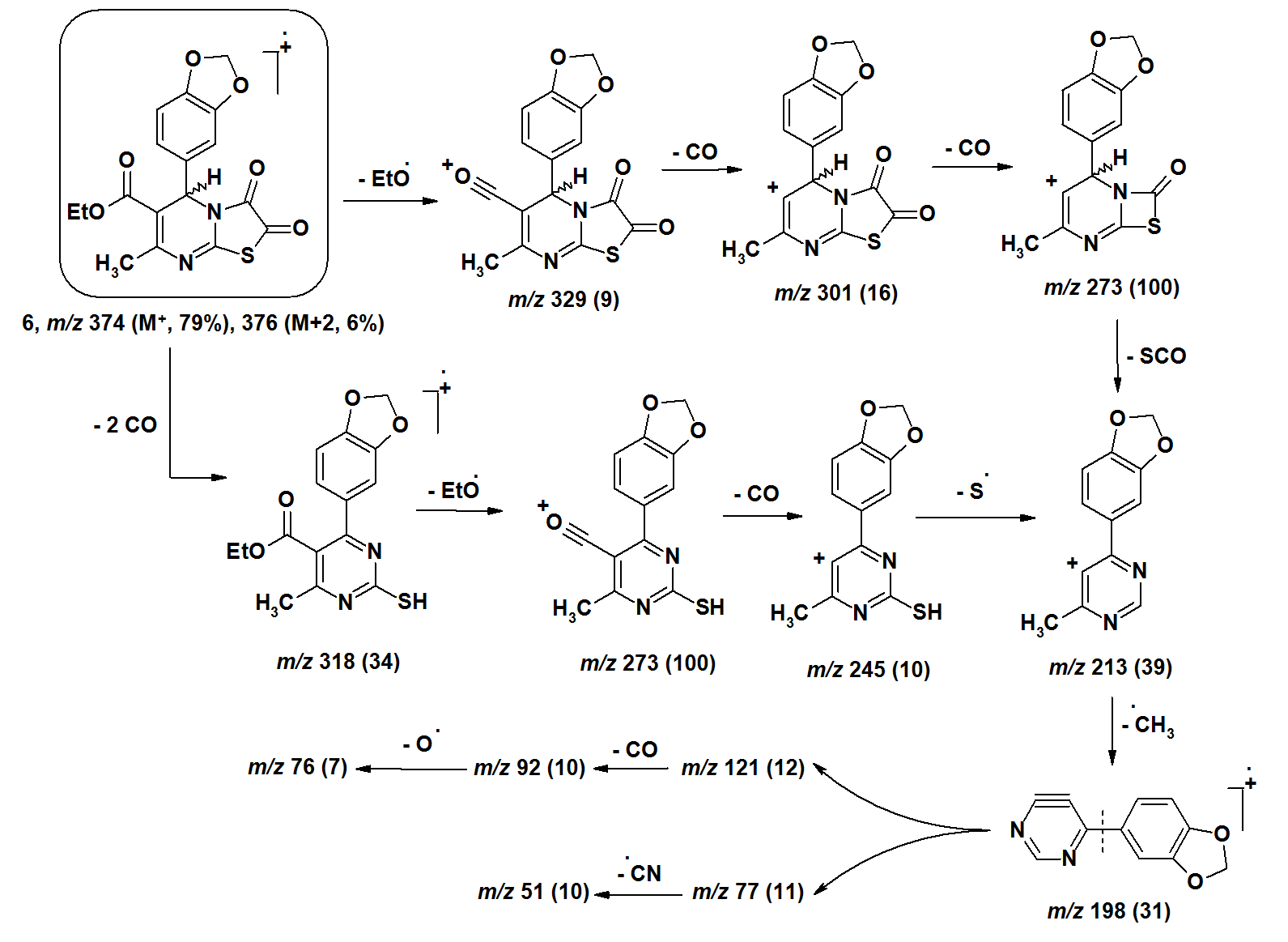 | Figure 5. |
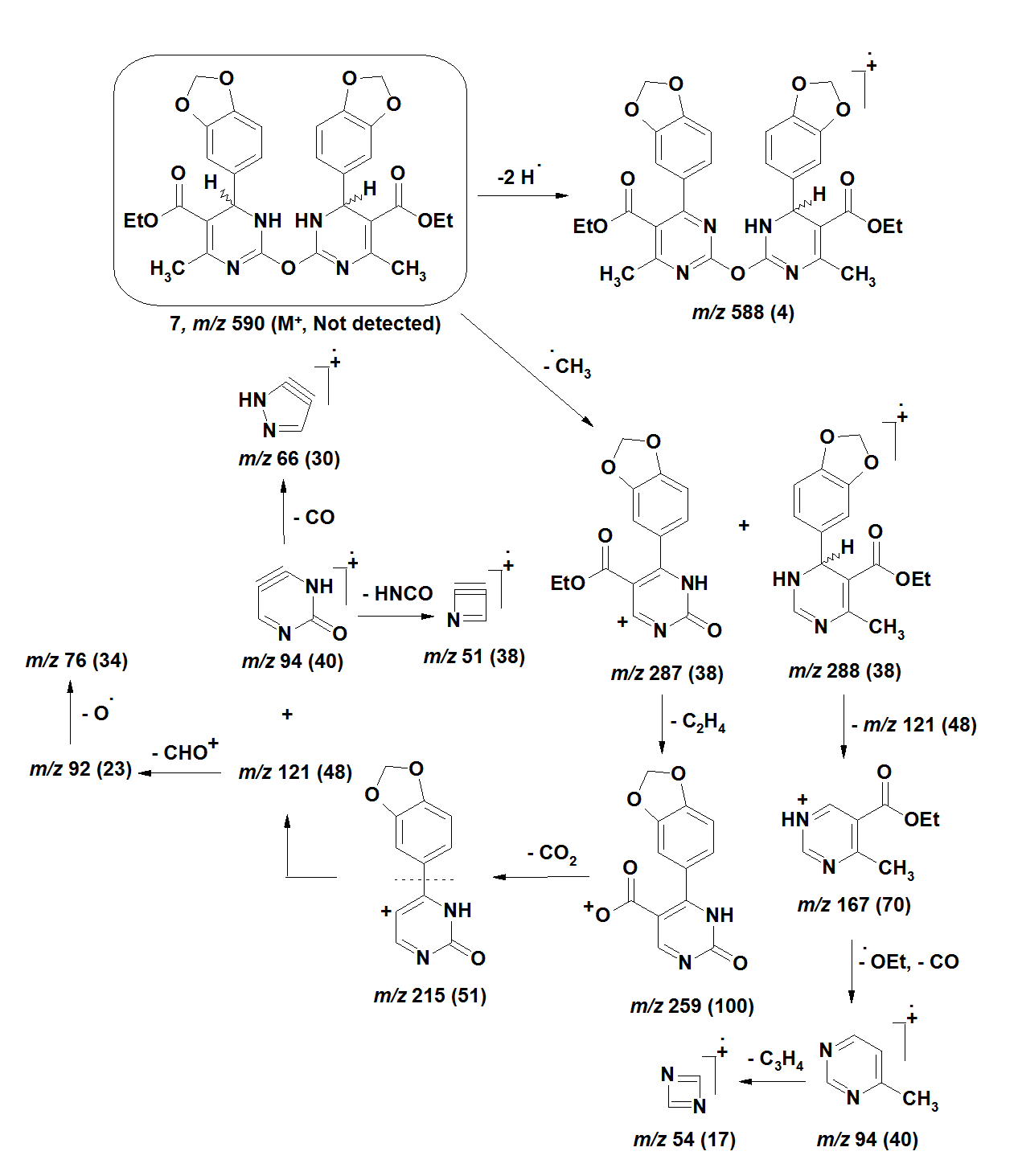 | Figure 6. |
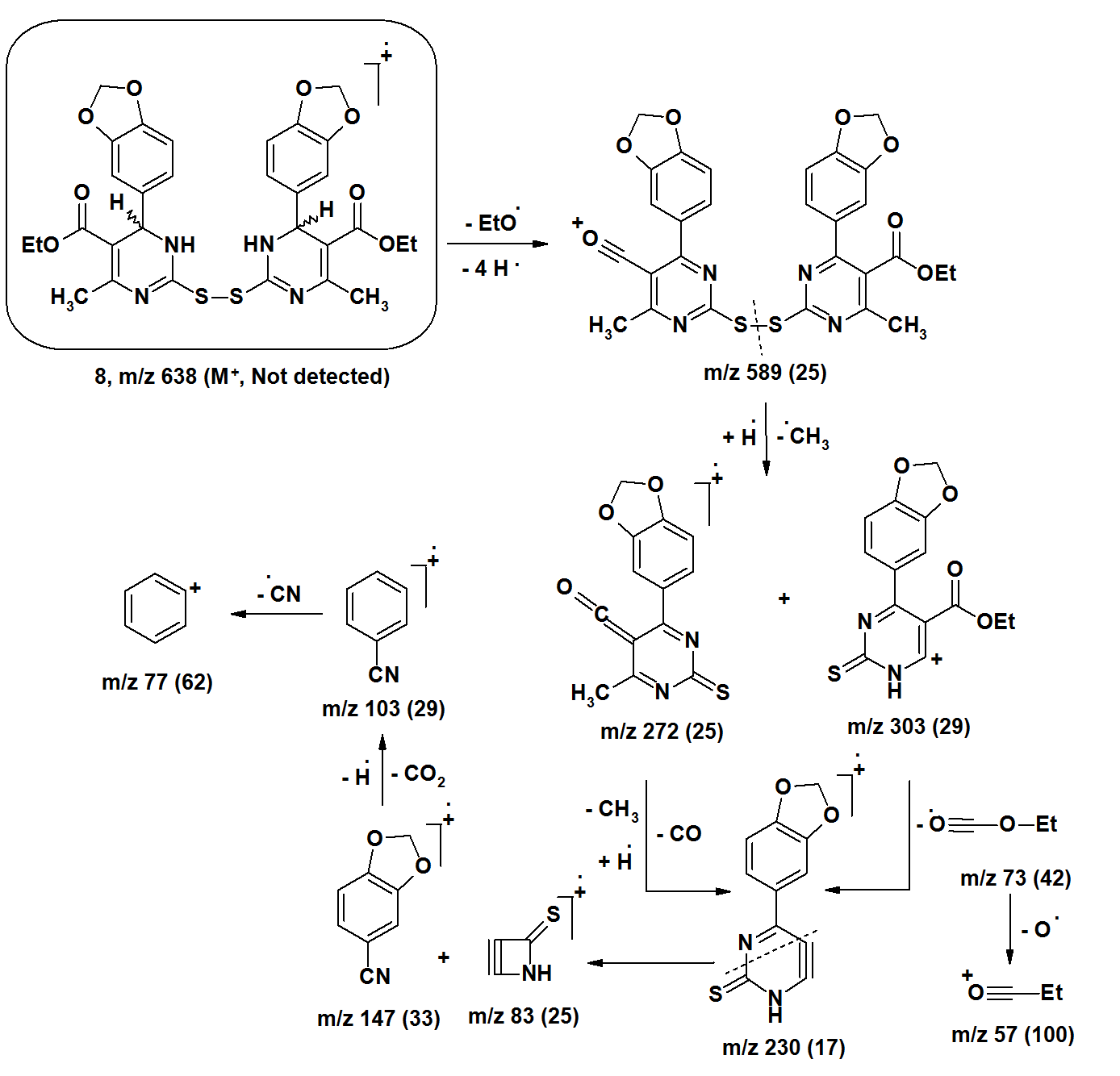 | Figure 7. |
4. Conclusions
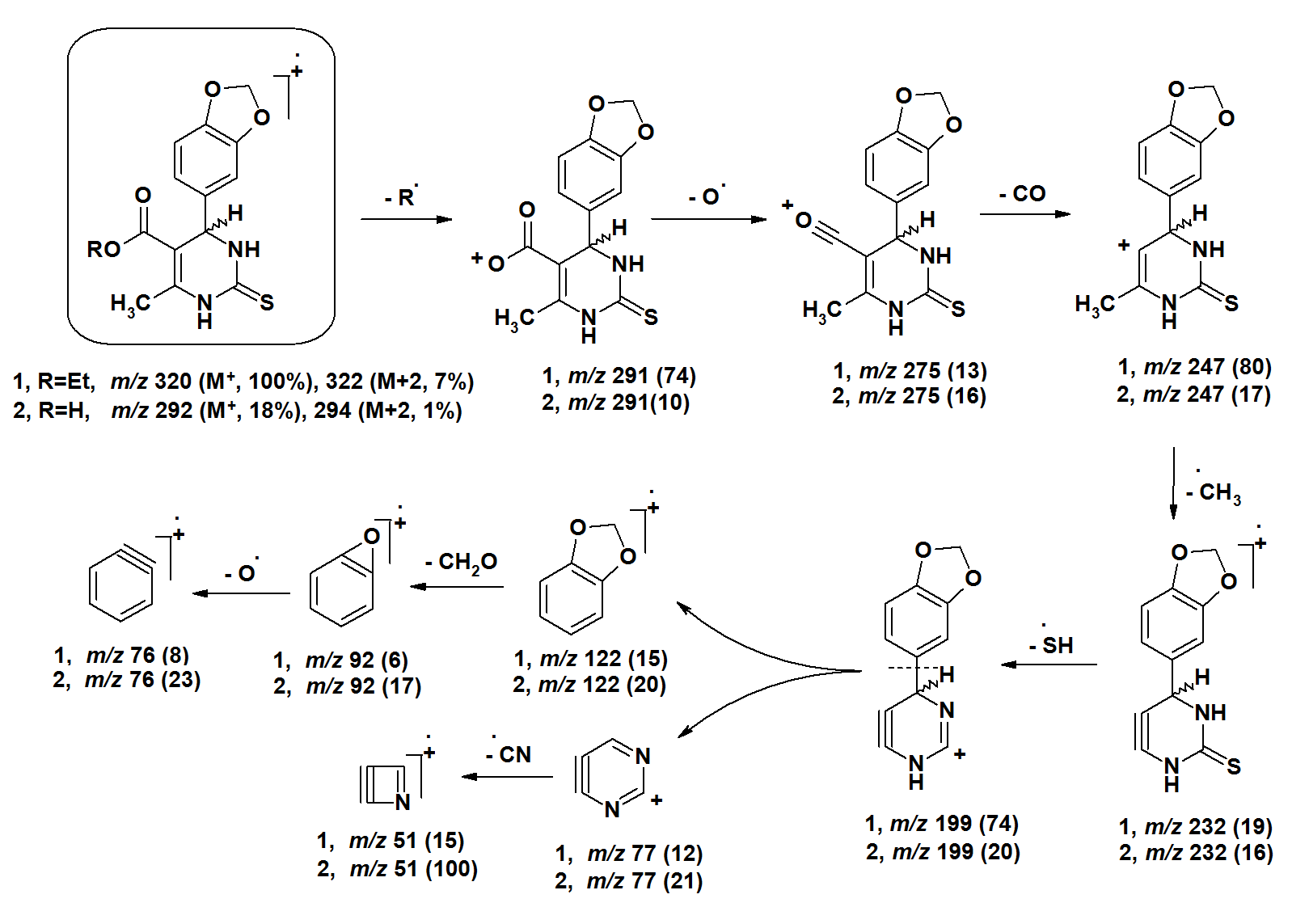
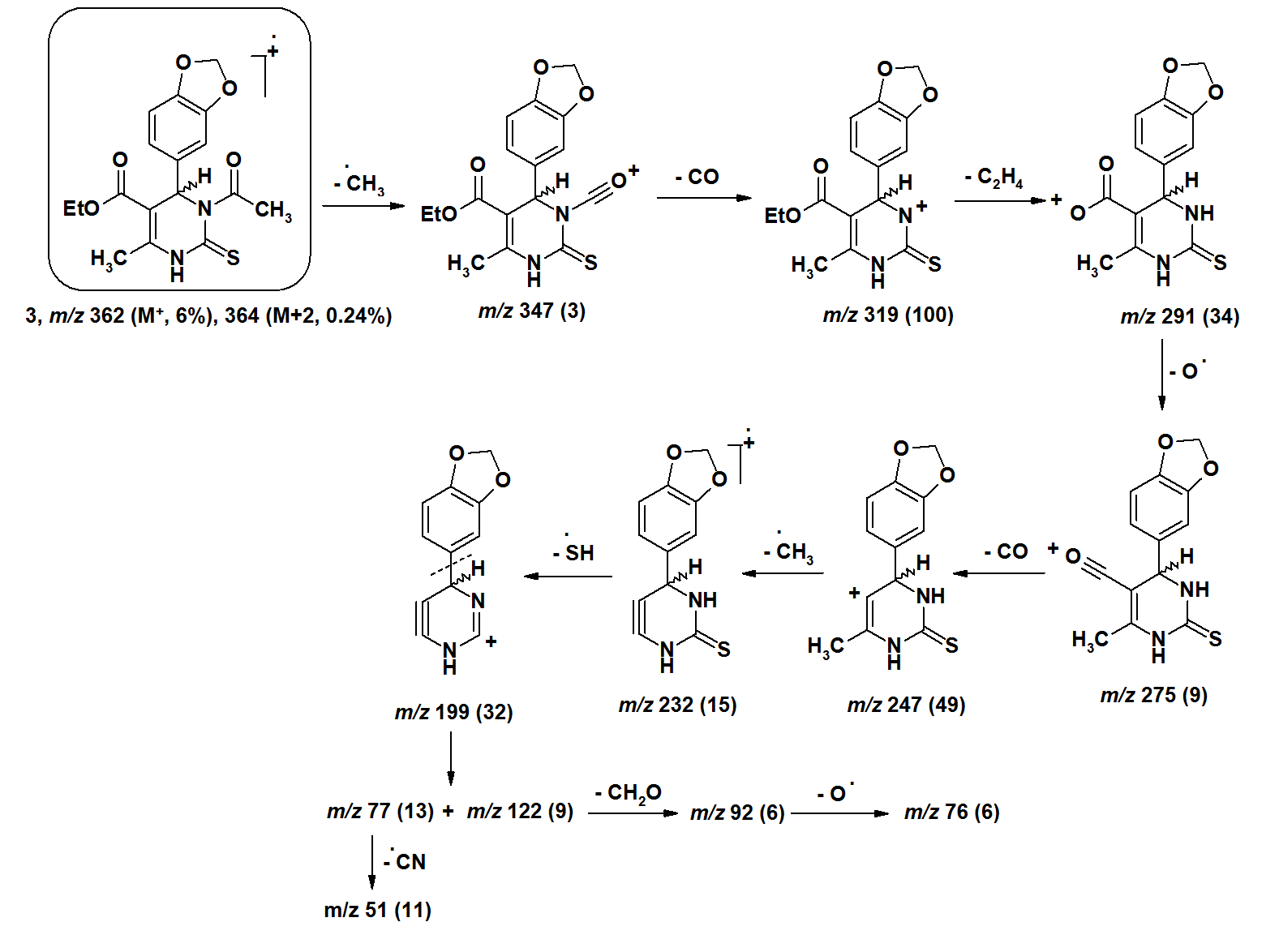
- The [M+•] ion of compounds 1–6 are low to high intensities (relative abundances 6–100%). Compounds 7 and 8 did not record their molecular ions. They underwent oxidation process followed by decomposition due to easy breakage of ether and dithioether bonds. Most of the studied compounds recorded the molecular ion peaks M+2 due to presence of sulfur isotopes. Compounds of each group take nearly the same fragmentation modes to give the corresponding fragments in similar relative abundance. The fragments obtained from compounds 1–3 are produced by elimination of side functional groups followed by fragmentation of the pyrimidine ring. The compounds 4–6 undergo fragmentation of the thiazole rings followed by fragmentation of the pyrimidine rings. This means the pyrimidine rings are more stable than the thiazole rings during the fragmentation process. Because of the difficult decomposition of the pyrimidinethione moieties in all compounds 1–8, they appear in the formation of the fragments that have molecular weight larger than m/z 77.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML