-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2014; 4(4): 79-87
doi:10.5923/j.ijmc.20140404.01
Crystallization of Synthetic Wollastonite Prepared from Local Raw Materials
Mohammed Maitham Obeid
College of Materials Engineering, Department of Ceramic Engineering, University of Babylon, Babylon, Iraq
Correspondence to: Mohammed Maitham Obeid, College of Materials Engineering, Department of Ceramic Engineering, University of Babylon, Babylon, Iraq.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Wollastonite (CaSiO3) has been synthesized by the solid state reaction method at a temperature range of 1050-1250℃ from local raw materials, e.g. silica sand and limestone as well as pure chemicals in the form of carbonate and quartz with and without B2O3 adding as a mineralized. The resulting products are investigated employing XRD and SEM techniques. β-wollastonite was obtained at 1050℃ and transformed to pseudowollastonite (α- CaSiO3) at 1150℃ due to the presence of B2O3. While the pure chemicals failed to give wollastonite at this range of temperature 1050-1150℃. As the temperature increased up to 1400℃, both experimental and standard samples have been melted.
Keywords: Wollastonite, XRD, SEM
Cite this paper: Mohammed Maitham Obeid, Crystallization of Synthetic Wollastonite Prepared from Local Raw Materials, International Journal of Materials and Chemistry, Vol. 4 No. 4, 2014, pp. 79-87. doi: 10.5923/j.ijmc.20140404.01.
1. Introduction
- Wollastonite is a calcium metasilicate that has a chemical formula of CaSiO3 with a theoretical composition of 48 wt.% CaO and 52 wt.% SiO2 [1-2]. Wollastonite is rarely found in the pure state and it contains an insignificant amount of harmful impurities in the form of manganese, magnesium, iron, strontium and titanium oxides. It occurs predominately as a contact metamorphic deposit formed between limestones and igneous rocks, often associated with garnet, diopside, epidoite, calcite and quartz [3-4]. Wollastonite is a polymorphic substance that occurs in three polymorphic forms; low temperature triclinic form [1T], monoclinic form or the so called para-wollastonite [2M] and the high temperature form pseudo-wollastonite which occurs in the pseudo-hexagonal form which is found rarely in nature. The conversion of the low temperature form to the high temperature form takes place at 1125℃ [5-7].Wollastonite is an extremely interesting but little studied material which has a combination of properties, such as lack of volatile constituents, fluxing characteristics, low dielectric constant, low dielectric loss, thermal stability, low thermal expansion and low thermal conductivity, hence is used in ceramic fabrication, medical material for artificial bones and dental roots, high frequency insulator, filler material in resins and plastics, paper, civil construction, metallurgy, paint and frictional products [8-15]. This situation has urged research workers to produce it synthetically [16]. Iraq has an abundance of limestone and silica sand resources with high purity contents of calcium oxide (CaO) and silicon dioxide (SiO2). Till now, nobody has used Iraqi limestone and silica to produce wollastonite synthetically.The present study aims to produce wollastonite at low sintering temperature from local raw materials e.g. silica sand and limestone which used as a source for CaO where they distributed abundantly in many regions of Iraq and compare it with those obtained from pure chemicals of CaCO3 and quartz with and without addition of B2O3.
2. Materials and Methods
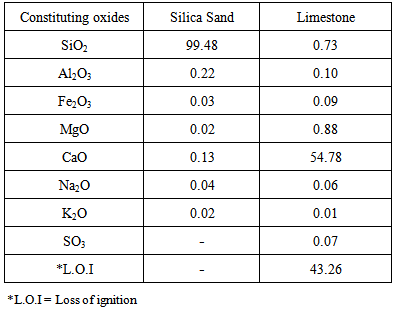
- Silica sand which is found abundantly in the western desert of Iraq with high content of (SiO2) and large deposits of limestone are available in different regions of Iraq where it obtained from the quarry of Wadi Al-Ubaiyidh. These two raw materials were washed several times with distilled water to get rid of any accompanying dust and undesired materials, dried and mixed with and without the addition of 2.24 wt% B2O3 in the form of H3BO3 to produce experiment wollastonite (Exp.). While extra pure calcium carbonate 98-100% (Thomas Baker, made in India) and silica>99% (Sibelco, made in Belgium) were used to produce the so called "standard wollastonite" (St.). The chemical analysis of both raw materials, e.g. silica sand and limestone is depicted in Table 1.
|
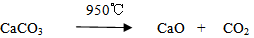 Fine powder of calcium oxide with off-white color was obtained. Calcium oxide (CaO) was mixed with SiO2 in 1:1 molar ratio with the addition of 4 wt% boric acid (H3BO3) to provide about 2.24 wt% of boron oxide (B2O3) that act as a mineralizer during the sintering process. Stoichiometrically, the amount of these materials was weighed out using analytical electric balance (M254A) with weighing sensitivity of about ± 0.0001 g to obtain a batch of 100 g as shown below:
Fine powder of calcium oxide with off-white color was obtained. Calcium oxide (CaO) was mixed with SiO2 in 1:1 molar ratio with the addition of 4 wt% boric acid (H3BO3) to provide about 2.24 wt% of boron oxide (B2O3) that act as a mineralizer during the sintering process. Stoichiometrically, the amount of these materials was weighed out using analytical electric balance (M254A) with weighing sensitivity of about ± 0.0001 g to obtain a batch of 100 g as shown below: Quicklime (CaO) = 47.22 g
Quicklime (CaO) = 47.22 g Silica sand (SiO2) = 50.54 g
Silica sand (SiO2) = 50.54 g Mineralizer (B2O3) = 2.24 gThe mixed powders with the addition of 300 ml of distilled water as a dispersive media and 2% PVA as a binder were wet milled by the planetary ball mill (SFM-1, QM-3SP2), which runs at 300 RPM for 15 hours to provide a homogenous fine mixture. The obtained mixture was oven dried for 24 hours at 80℃, then finely crushed using pestle and mortar. The aim of milling the mixture in water media is to hydrate the calcium oxide which reacts with water rapidly producing calcium hydroxide and heat, according to the following reaction:
Mineralizer (B2O3) = 2.24 gThe mixed powders with the addition of 300 ml of distilled water as a dispersive media and 2% PVA as a binder were wet milled by the planetary ball mill (SFM-1, QM-3SP2), which runs at 300 RPM for 15 hours to provide a homogenous fine mixture. The obtained mixture was oven dried for 24 hours at 80℃, then finely crushed using pestle and mortar. The aim of milling the mixture in water media is to hydrate the calcium oxide which reacts with water rapidly producing calcium hydroxide and heat, according to the following reaction: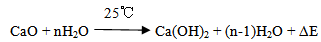 Excessive water was removed by drying process at 150℃ for 24 hours according to the following.
Excessive water was removed by drying process at 150℃ for 24 hours according to the following.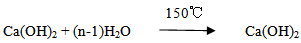 The mixture of calcium hydroxide Ca(OH)2, silica (SiO2) and H3BO3 were calcined at 710℃ in an alumina crucible in air atmosphere for 3 hours, where calcium hydroxide start to decompose at temperatures above 580℃ to give calcium oxide while water is vaporized according to the following reaction:
The mixture of calcium hydroxide Ca(OH)2, silica (SiO2) and H3BO3 were calcined at 710℃ in an alumina crucible in air atmosphere for 3 hours, where calcium hydroxide start to decompose at temperatures above 580℃ to give calcium oxide while water is vaporized according to the following reaction: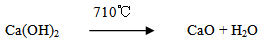 At this temperature (710℃), boric acid (H3BO3) is decomposed to boron oxide (B2O3) which tend to lower the sintering temperature of the mixture. Particle size was measured using a laser particle size analyzer (Bettersize2000) to give an average particle size of about 5.47 μm According to D10, D50 and D90 as shown in Figure 1.
At this temperature (710℃), boric acid (H3BO3) is decomposed to boron oxide (B2O3) which tend to lower the sintering temperature of the mixture. Particle size was measured using a laser particle size analyzer (Bettersize2000) to give an average particle size of about 5.47 μm According to D10, D50 and D90 as shown in Figure 1.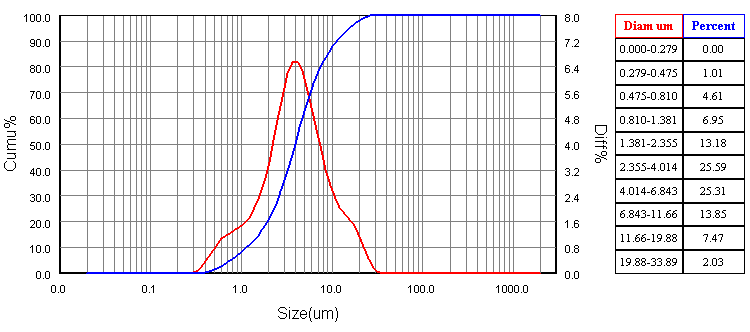 | Figure 1. Particle size analysis of the mixed experimental powder [6] |
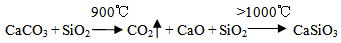 The mixed powders with the addition of 300 ml of ethanol as a dispersive media and 2% PVA as a binder were wet milled by the planetary ball mill (SFM-1, QM-3SP2), which runs at 300 RPM for 15 hours to provide a homogenous fine mixture. The wet mixture was oven dried at 100℃ for 24 hours to be sure that all moistures were removed. Particle size was measured using a laser particle size analyzer (Bettersize2000) to give an average particle size of 14.68 μm as shown in Figure 2.
The mixed powders with the addition of 300 ml of ethanol as a dispersive media and 2% PVA as a binder were wet milled by the planetary ball mill (SFM-1, QM-3SP2), which runs at 300 RPM for 15 hours to provide a homogenous fine mixture. The wet mixture was oven dried at 100℃ for 24 hours to be sure that all moistures were removed. Particle size was measured using a laser particle size analyzer (Bettersize2000) to give an average particle size of 14.68 μm as shown in Figure 2.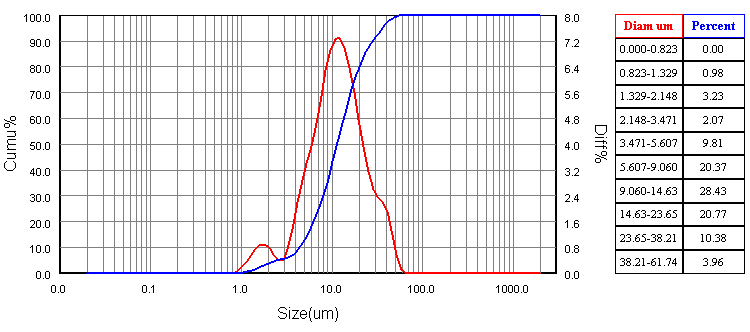 | Figure 2. Particle size analysis of the so called mixed "standard" powder |
3. Results and Discussion
- The crystallization behavior of both experimental and standard wollastonite is investigated by XRD at different temperatures. At 1050℃, it seems that almost all peaks are related to low temperature monoclinic wollastonite β-CaSiO3 of a maximum relative intensity (100%) at a diffraction angle of 2Ɵ equal to 29.898° (2.98 A°), with little amount of unreacted quartz. Meanwhile, some portion of SiO2 had been converted into its high temperature polymorph tridymite. This transformation was duly supported by the fact that quartz starts converting into tridymite at 867℃ [17]. While in the so called "standard work", unreacted quartz appears as a major phase at 2Ɵ equal to 26.566° (3.35 A°). It can be noted that calcium oxide reacts with silica to give larnite (Ca2SiO4) and β-wollastonite with lower peak intensity as shown in Figure 3.
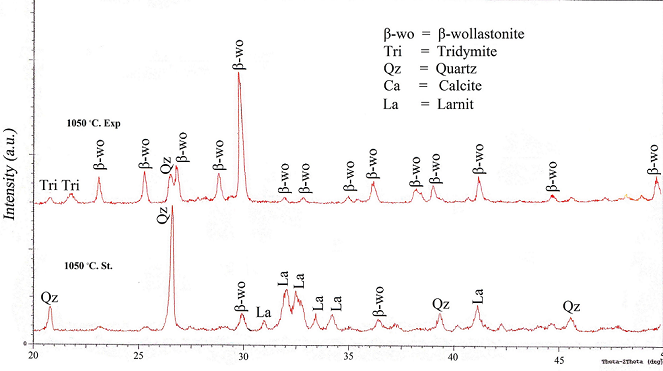 | Figure 3. XRD patterns of the so called "standard" and experimental work at 1050℃ |
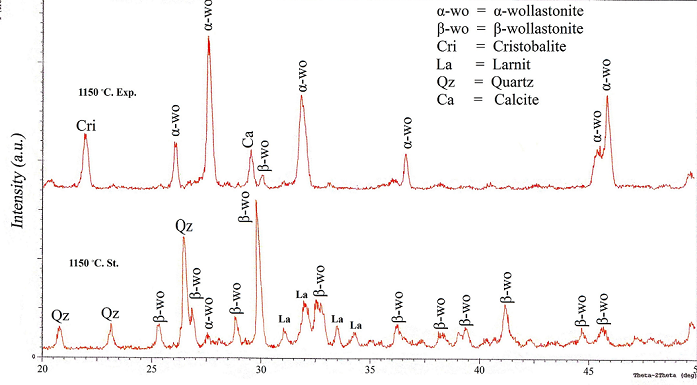 | Figure 4. XRD patterns of the so called "standard" and experimental work at 1150℃ |
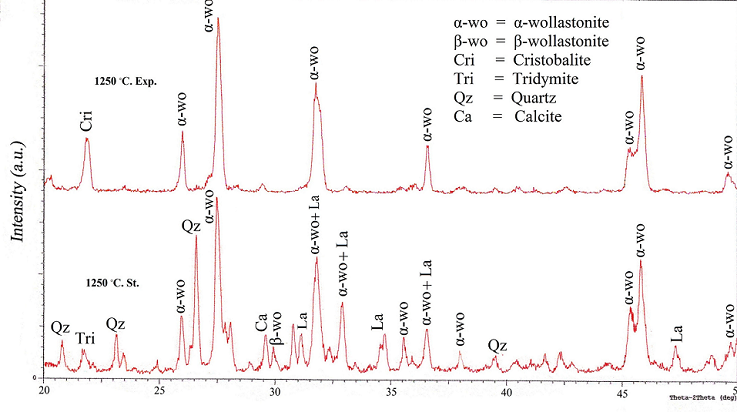 | Figure 5. XRD patterns of the so called "standard" and experimental work at 1250℃ |
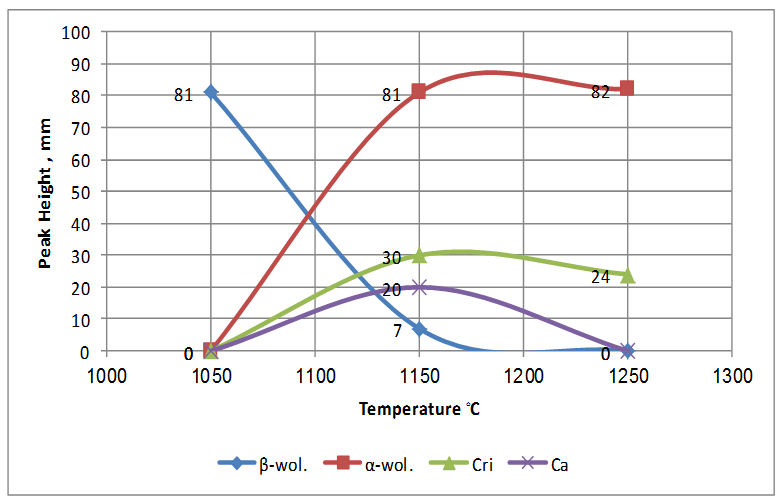 | Figure 6. Crystallization behavior of experimental work with respect to the sintering temperatures |
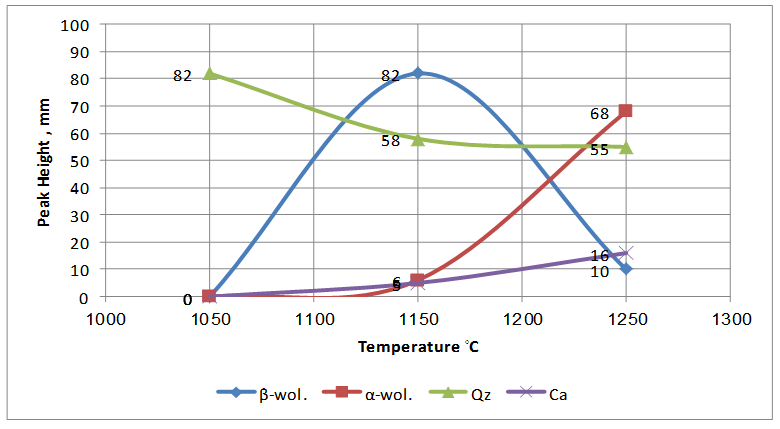 | Figure 7. Crystallization behavior of the so called "standard work" with respect to the sintering temperature |
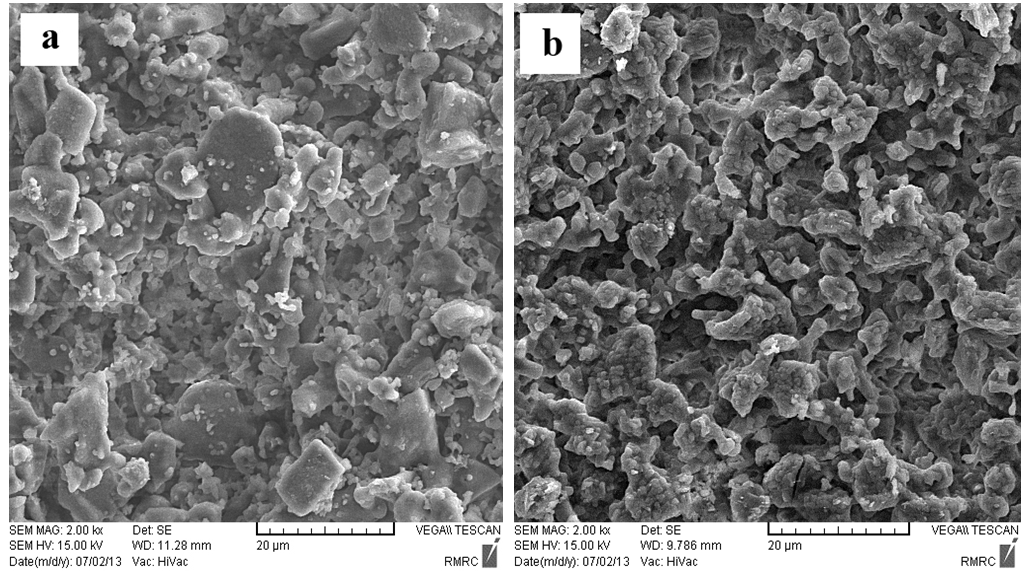 | Figure 8. SEM micrographs for (a) the so called "standard" and (b) experimental work sintered at 1050℃ for 2 h [6] |
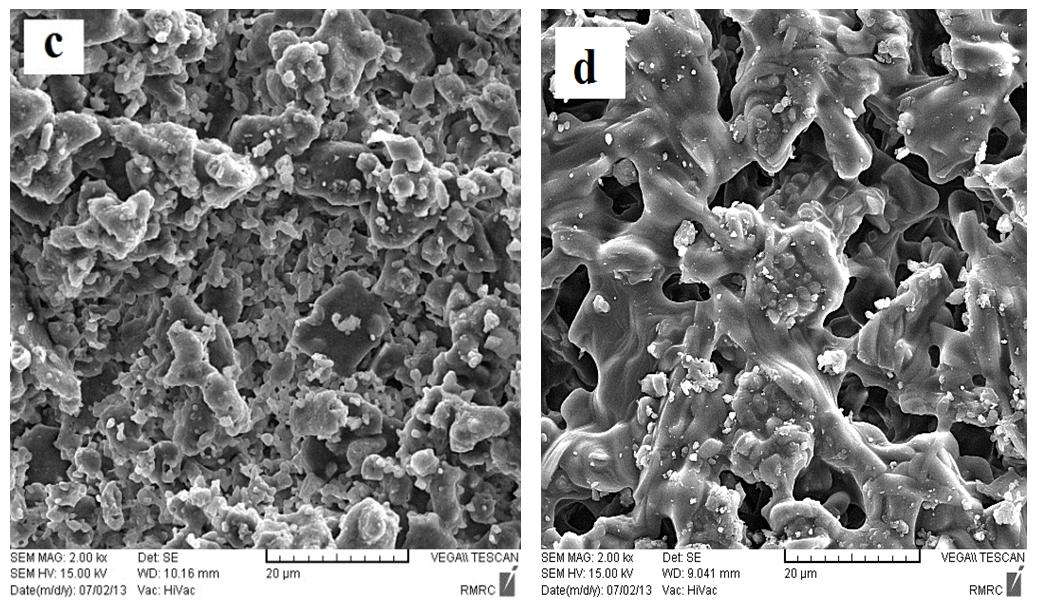 | Figure 9. SEM micrographs for (c) the so called "standard" and (d) experimental work sintered at 1150℃ for 2 h [6] |
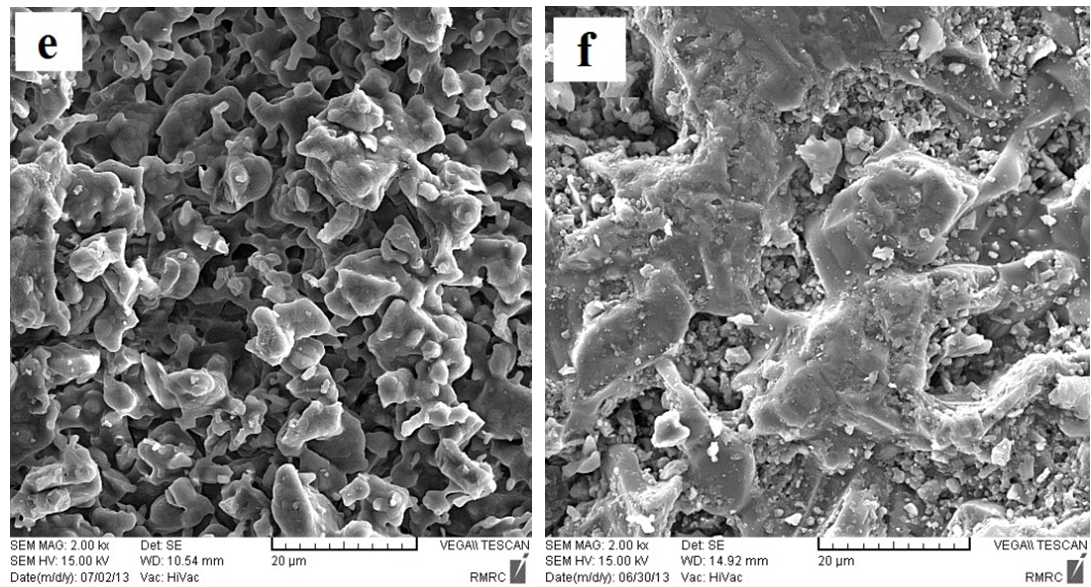 | Figure 10. SEM micrographs for (e) the so called "standard" and (f) experimental work sintered at 1250℃ for 2 h [6] |
4. Conclusions
- Synthetic wollastonite was successfully prepared from limestone and silica sand with the addition of 2.24 wt. % B2O3 at low temperature of 1050℃ for β-wollastonite and of 1150℃ for α-wollastonite with relatively porous structure. Pure chemicals failed to give β- and α- forms at low temperature with highly porous structure as confirmed by XRD and SEM. Increasing in firing temperature to 1400℃ causes melting of both standard and experimental samples.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML