-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2014; 4(3): 69-77
doi:10.5923/j.ijmc.20140403.04
Effect of pH on Electrochemical Behaviour of a Ni-Cr-Mo Commercial Dental Alloy in Chloride Medium
Luís Gustavo Costa de Castro1, Sérgio Fernando Nunes Júnior1, José Wilson de Jesus Silva2, Eduardo Norberto Codaro1, Heloisa Andréa Acciari1
1Physics and Chemistry Department, Faculty of Engineering, Guaratinguetá, Brazil
2Teresa D'Avila Integrated Faculties, FATEA, Lorena, Brazil
Correspondence to: Heloisa Andréa Acciari, Physics and Chemistry Department, Faculty of Engineering, Guaratinguetá, Brazil.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Basic metal alloys, as Ni-Cr-Mo, have been widely used in the manufacture of fixed and removable prostheses, due to their desirable physical and mechanical properties. However, these alloys undergo an oxidation in the oral environment, which causes metal ions release in the body, leading to harmful systemic effects for human health. The purpose of this study is to assess the electrochemical behavior of a Ni-Cr-Mo commercial dental alloy, in a physiological environment that simulates the aggressiveness of oral cavity (0.9% NaCl solution) with its pH varying from 2.0 to 6.0. The corrosion behavior was assessed by electrochemical measurements which are commonly applied in metals’ corrosion study, with quantitative parameters to estimate corrosion resistance. In the open circuit potential curves, it was observed a typical behavior of passive state, mainly at pH 4.0 and 6.0. On the potentiodynamic curves it was observed that the passive current density increased and the re-passivation potential decreased with diminishing pH, suggesting that the re-passivation does not occur at pH 2.0. Chronoamperometric curves, as well as EIS spectra shapes, were consistent with these analyses. The results obtained from different methods of analyses indicate that corrosion resistance can be affected by pH changes in a saline medium.
Keywords: Ni-Cr alloys, Corrosion, Chloride Ions, Electrochemical Techniques
Cite this paper: Luís Gustavo Costa de Castro, Sérgio Fernando Nunes Júnior, José Wilson de Jesus Silva, Eduardo Norberto Codaro, Heloisa Andréa Acciari, Effect of pH on Electrochemical Behaviour of a Ni-Cr-Mo Commercial Dental Alloy in Chloride Medium, International Journal of Materials and Chemistry, Vol. 4 No. 3, 2014, pp. 69-77. doi: 10.5923/j.ijmc.20140403.04.
Article Outline
1. Introduction
- Tooth structure loss has been an issue for a long time in dentistry due to aesthetics and functional problems that affect the health and life quality of patients. Many studies have contributed to new materials development which can restore features and the natural functions of teeth. Basic metal alloys, such as Ni-Cr-Mo, have emerged in the dental market as a practical result of this study being widely used in the manufacture of fixed and removable prostheses, particularly in developing countries, due to their desirable physical and mechanical properties, and also because they are easily processed and inexpensive [1, 2].However, these alloys undergo an oxidation in the oral environment, leading to metal ions release in the body, with systemic effects which are harmful to human health, leaving doubts as to its biocompatibility [1, 3]. Ni, for example, has an allergenic feature and Cr, when present in its chromate form, is highly toxic [1]. Therefore, new formulations of Ni-Cr-Mo alloys were evaluated.Since Ni2+ release causes allergic reactions, less noble alloying elements can also be dissolved causing diseases from a cumulative process that will be manifested only after many years wearing the prosthesis. The quickness in which an ion is released into the corrosive medium is a function of the alloy composition and the solubility of the corrosion products. Therefore, a higher ions release leads to a higher risk of undesirable reactions in the mouth. Even in metal-ceramic restorations, when a ceramic material is fused to the alloy, this problem is not discarded due to atoms inter-diffusion at the interface metal/ceramic interface reaching temperatures of about 1000℃ [4].Corrosion researchers concluded that the Ni amount released into the solution depends on a combination of factors, such as Cr and Mo contents, where these factors are responsible for a decrease in the Ni release due to a formation of Cr and Mo oxides, predominantly Cr2O3 and MoO2, by means of evidence of higher localized corrosion susceptibility in dendritic structures of Mo-free Ni-Cr alloys [5-7].In the oral cavity, these structures are exposed to a chemically adverse environment, due to a high concentration of chloride ions in the saliva composition and also to constant changes in pH and temperature. This process may be enhanced through abrasion from food intake and brushing. An aggravating factor is that, once it is placed in the mouth, it must remain subjected to mechanical stress and corrosion for a long time. In an attempt to simulate these conditions, the electrochemical behavior of these alloys has been commonly assessed in artificial saliva or in solutions with concentrations of chloride ions which are similar to that found in the chemical composition of natural saliva [5, 8-12].In this paper, it is proposed to assess the electrochemical behavior and corrosion resistance of a dental Ni-Cr-Mo alloy in a physiological environment that simulates the aggressiveness of the oral cavity (0.9% NaCl), by varying the pH from 2.0 to 6.0. Electrochemical techniques commonly applied to the study of metallic corrosion provided quantitative parameters to estimate corrosion resistance.
2. Materials and Methods
- It was used a dental alloy widely commercialized with the following nominal composition (wt.%): Ni-62.01; Cr-32.65; Mo-1.02; Co-3.00; other-1.01. Prior to electrochemical measurements, the sample was mechanically polished using SiC polishing paper and finished with alumina suspension (1 µm). Thereafter, it was ultra-sonically rinsed in distilled water for 900 s. Subsequently the sample was electro-etched in 20% (wt.%) at 2.0 V for 3 s and examined using a Nikon Epiphot 200 optical microscope. For corrosion experiments, the alloy surface was polished and rinsed as previously described. In this new step, an electro-etching was not performed. Electrochemical measurements were achieved in a three electrodes cell containing an electrolyte which is similar to physiological serum (0.9% aqueous physiological solution) at different values of pH (2.0, 4.0, and 6.0). The counter electrode was a Pt foil and the reference electrode was an AgCl/Cl-. Solutions were prepared using an analytical grade reagent and were not deaerated. Experiments were carried out at room temperature (25o C).Electrochemical measurements were made with a PGSTAT302 potentiostat (Eco. Chemie B. V., Utrecht, Netherlands), that included corrosion potential, cyclic polarization (at 0.001 V s-1 from OCP until +1.0 V) and chronoamperometric curves (at +0.3 V for 450 s). Additionally, impedance measurements were carried out with a 10 mV rms perturbation in a frequency ranging from 100 kHz to 0.01 Hz, ten points per frequency decade, fitted by FRA software (Frequency Response Analyser, Eco. Chemie B. V., Utrecht, Netherlands) version 4.9. A minimum of two replicates was carried out for each type of test.
3. Results
- Figure 1 shows the microstructure of the investigated dental Ni-based alloy after its electro-etching. It reveals a microstructure characterized by porous of different sizes and shapes randomly distributed in a typical dendritic arrangement (light grey) in a solid solution matrix (dark grey).
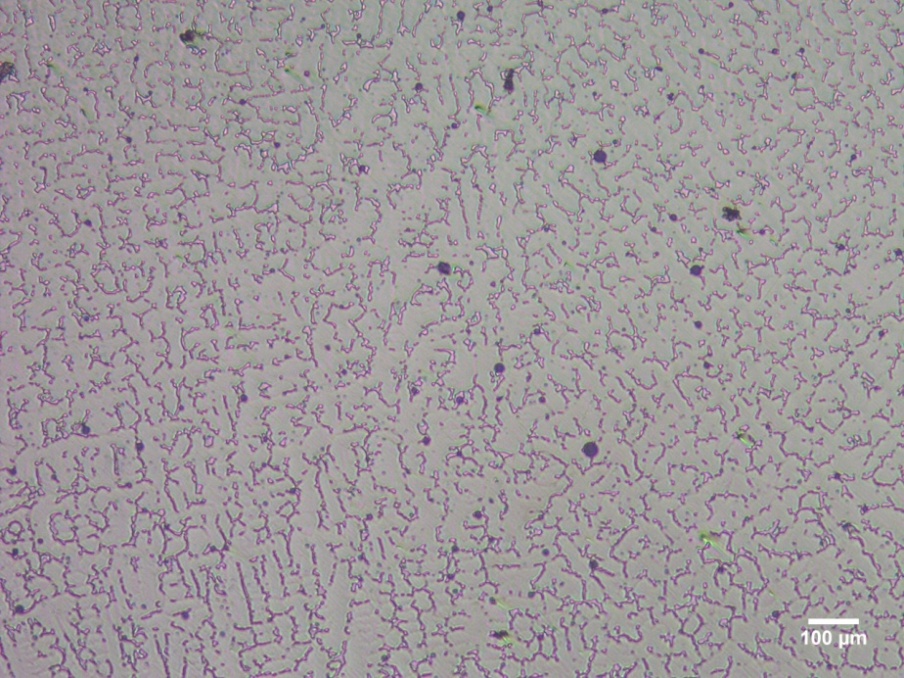 | Figure 1. Optical micrograph of Ni-Cr-Mo alloy |
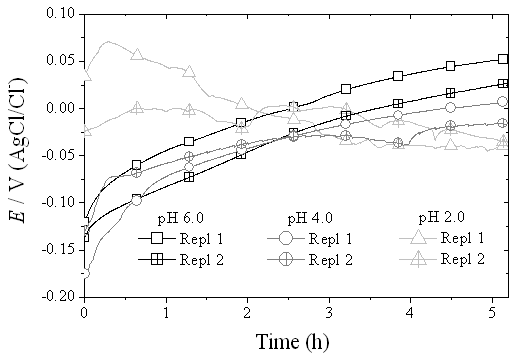 | Figure 2. OCP curves of the Ni-Cr-Mo alloy in 0.9% NaCl medium at different values of pH |
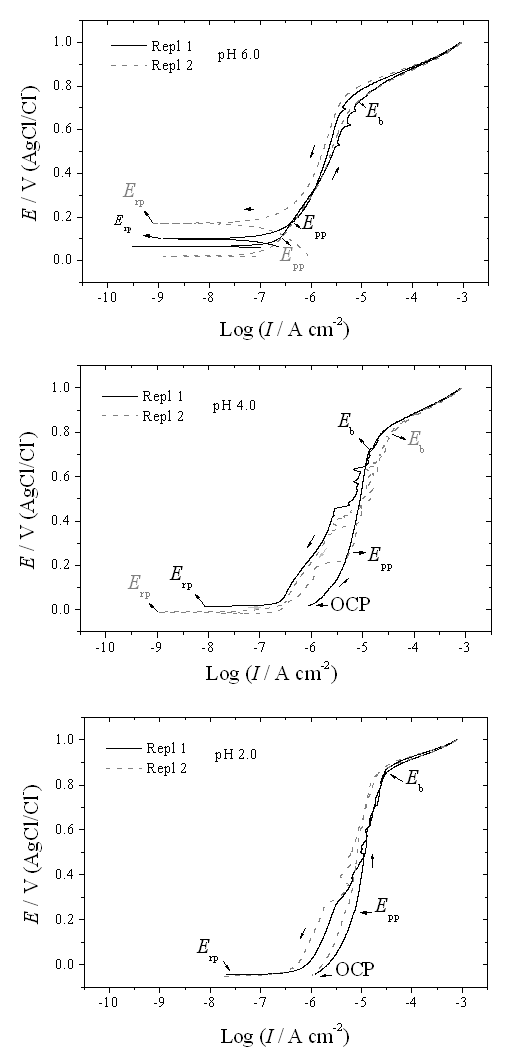 | Figure 3. CP curves of the Ni-Cr-Mo alloy in 0.9% NaCl medium at different values of pH |
|
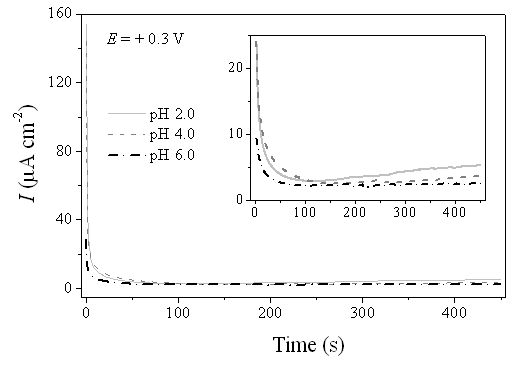 | Figure 4. Chronoamperometric curves recorded at +0.3 V (AgCl/Cl-) for the Ni-Cr-Mo alloy in 0.9% NaCl medium at different values of pH |
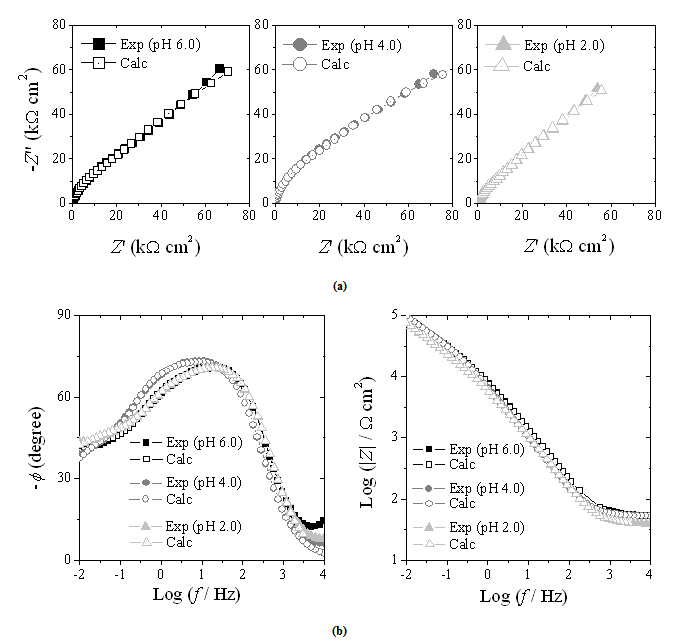 | Figure 5. EIS spectra obtained for the Ni-Cr-Mo alloy in 0.9% NaCl medium at different values of pH: (a) complex plane format; (b) Bode format |
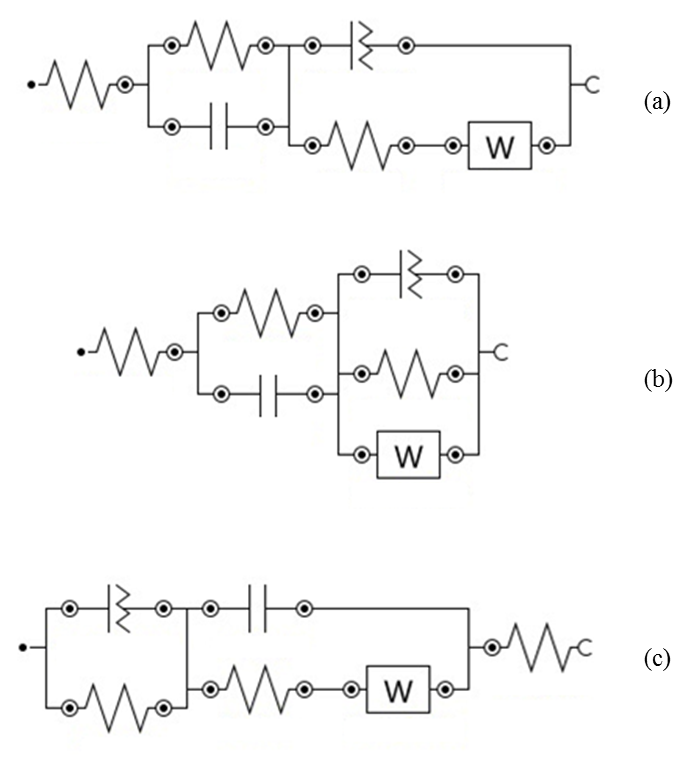 | Figure 6. Equivalent electrical circuit models used for the fitting of EIS spectra: (a) R1(R2C1)(Q1R3W); (b) R1(R2C1)(Q1[R3W]); (c) (Q1R1)(C1[R2W])R3 |
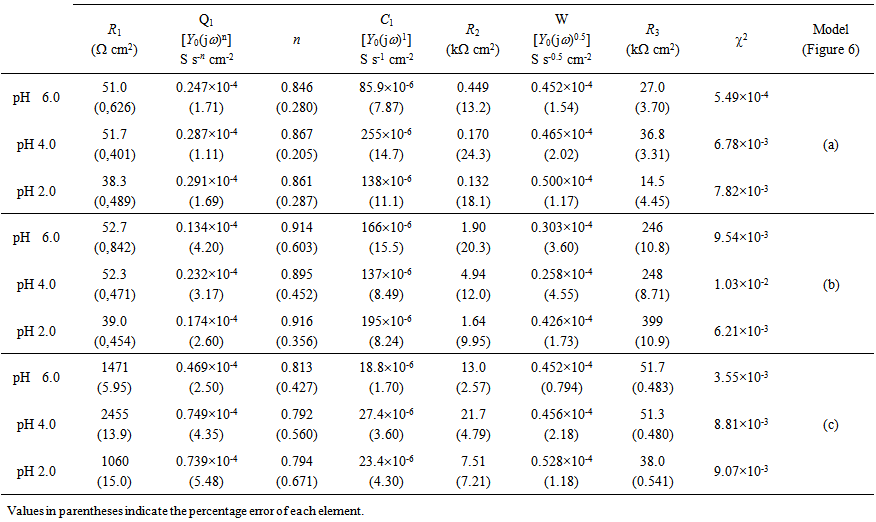 | Table 2. Fitting results of the EIS spectra obtained for the Ni-Cr-Mo alloy in 0.9% NaCl medium at different pH values |
4. Discussion
- Although Ni-Cr-Mo alloys are widely disseminated in the dental market, very few publications are found in literature regarding corrosion studies in a chloride medium. The most recent works with OCP, CP, and EIS measures generally correlate different compositions of Ni-Cr-Mo alloys with corrosion resistance, so that works reporting the influences of variations in the composition, temperature, or pH of the saline medium do not have any precedents.Reclaru et al. [16] also point out to the lack of epidemiological studies on the correlation between Ni-Cr alloys in the mouth and allergies due to contact with Ni because its released quantity is greater than the limits imposed by the European Union, whereas these alloys were effectively banned in countries such as Sweden and Denmark because of it. Despite the imposed restrictions, the use of Ni-Cr alloys is increasing even more, which raises the need for more conclusive studies on its effect in the human organism for many years. One of the criteria assessed in these studies takes into account the association of Eb to a better behavior regarding corrosion resistance. In the comparison of different compositions of Ni-Cr-Mo alloys with those of noble metals, for example, the lowest values of Eb were recorded for the Ni-Cr-Mo, which means a greater possibility of corrosion with more Ni release [15].Basic metals alloys, used as biomaterials, are commonly covered with a thin layer of oxides, whose composition would vary according to alterations of the medium due to continuous processes of localized dissolution and passivation, even though the film is macroscopically stable. Nagai et al [17] characterized the surface covered with an oxide film of a Co-Ni-Cr-Mo alloy immersed in Hanks solution, and found oxides of all constituent metals, in addition to a large amount of OH-, especially in the outer layers of the film. The authors also found that the Cr is distributed more on the inner layer and the Ni on the outer surface of the oxides film.Zhang et al. [18] characterized the properties of the passive film on a Ni-Cr-Mo alloy. These authors obtained a double layer structure, one being inner, Cr3+ ion-enriched, and the other one external containing hydrated oxides formed by the hydrolysis of the released cations of the innermost layer. As the potential increases within the passive range during the electrode polarization, the film’s composition changes because the oxidative dissolution of the innermost layer takes place instead. Moreover, the oxidation of Cr3+ into Cr4+ on the innermost layer of the film during the transpassive range and its resulting dissolution leads to a loss of Cr2O3 on this layer, and to a destruction of the passivity.In the case of passive alloys, corrosion takes place due to variations in chemical composition or properties of oxide film when in contact with the corrosive medium. In the mouth, this process is continuous due to the abrasion of food and brushing [1]. Still with respect to the characterization of the film, no attempt to adjust EIS spectra was successful using models of simple equivalent circuit without Warburg element inclusion, referenced in this work to take into account the shortcomings of the film, which allows the transport of ionic species through the same via ionic vacancies or interstitial cations with effects on the diffusion component represented by a straight segment in the low frequency region of the spectra [5]. Based on the EIS cited studies and on the results of this study, it was found that the properties of the passive film on the Ni-Cr-Mo alloy were determinant in the corrosion resistance.
5. Conclusions
- In the corrosion analysis of the Ni-Cr-Mo alloy in 0.9% NaCl, it was found a passive behavior under the assessed conditions. The passive film was interpreted in terms of a double layer structure, an inner one being more compact and outer one more porous. A diffusion process through the pores can be a way to release nickel ions release. With decreasing pH, a higher difficulty of the passive film to regenerate was observed, which can be due to changes in the morphology and structure of film in different media. The presented data clearly show that combined electrochemical techniques are very suitable for corrosion resistance assessment of dental alloys, through a good correlation between the results obtained from different methods of analysis.
ACKNOWLEDGEMENTS
- Acknowledgements are to PROPe/UNESP and FUNDUNESP for financial support. The authors would like to acknowledge the English language review, realized by FDCT – Foundation for Scientific and Technological Development.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML