-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2014; 4(3): 65-68
doi:10.5923/j.ijmc.20140403.03
Graft Copolymerization of N-vinyl Caprolactame onto Chitosan
V. O. Kudyshkin, A. M. Futoryanskaya, R. Yu. Milusheva, N. D. Kareva, S. Sh Rashidova
Institute of Polymer Chemistry and Physics Academy of science of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: V. O. Kudyshkin, Institute of Polymer Chemistry and Physics Academy of science of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
In the present work we studied the graft radical copolymerization of N-vinyl caprolactame onto chitosan, synthesized from the chitin of the silkworm pupae, in the presence of the potassium persulfate as initiator. The influence of the polymer, monomer and initiator concentration, as well as the temperature on the copolymer composition and the effectiveness of grafting are discussed.
Keywords: Chitosan, N-vinyl caprolactame, Potassium persulfate, Graft radical copolymerization, Copolymers composition
Cite this paper: V. O. Kudyshkin, A. M. Futoryanskaya, R. Yu. Milusheva, N. D. Kareva, S. Sh Rashidova, Graft Copolymerization of N-vinyl Caprolactame onto Chitosan, International Journal of Materials and Chemistry, Vol. 4 No. 3, 2014, pp. 65-68. doi: 10.5923/j.ijmc.20140403.03.
1. Introduction
- Chitosan (CS) is one of the most perspective polymers, as it is biocompatible, biodegradable, non-toxic and does not form threat for the environment. One of the most interesting approaches to CS chemical modification is obtaining grafted copolymers on their basis. Of particular interest are grafted copolymers of N-vinyl caprolactame (N-VCL) on chitosan, which can dissolve in water, have a stimulus responsive properties and antimicrobial activity. The authors [1, 2] provide a synthesis of grafted copolymers CS – poly N-VCL. The synthesis was performed in several stages. At the first stage the radical homopolymerization of N-VCL in the presence of chain transfer agent was carried out. Then conducted a grafting of the poly N-VCL chains onto CS account for the reaction terminal carboxyl group poly N-VCL and amino group of CS. Another approach is graft copolymerization of the vinyl monomer onto CS in the presence of the redox initiators [3-6]. Recently, we have established the fact of the grafted copolymers formation on the basis of crab chitosan and N-vinyl caprolactame [7]. For the countries of Central Asia are the interest of the study of chitosan derived synthes from the chitin of the silkworm pupae, which differs from the crab CS both in composition and molecular weight. In Uzbekistan has been developed the technology of obtaining chitin and chitosan. Raw materials for their production are the pupa of the silkworm, which are the waste of silk production [8].In connection with this, in the present work for the first time carried out grafted radical copolymerization N-VCL on CS of silkworm pupae chitin in the presence of potassium persulfate (KPS). The influence of the concentrations of CS, N-VCL, KPS, and temperature on the composition of obtained copolymers and the effectiveness of grafting are discussed.
2. Experimental
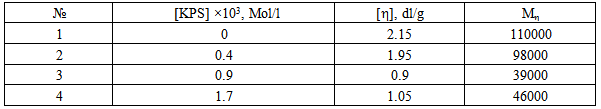
- For the experiments used the CS, synthesized from the chitin of the silkworm pupae, at the Institute of polymer chemistry and physics of the Academy of Sciences of Uzbekistan. CS has the following technical specifications: nitrogen content = 8.25%, degree of deacetylation = 77%, The intrinsic viscosity [η] = 2.15 dl/g in a solvent 0.25M CH3COOH+0.25M CH3COONa at 25℃. Technical characteristics of chitosan comply with the standard ТSh 88.2-13:2011 (Uzbekistan). The molecular weight of CS calculated by the equation [η] = 1.4•10-4×Мη0.83 [8]. The molecular weight Мη =110000.N-VCL monomer was manufactured at the Institute of Polymer Chemistry and Physics, Academy of Sciences of Uzbekistan, using acetylation of caprolactam. The obtained monomer was rectified by two vacuum distillations. Purity of the distilled N-VCL was tested using the method of reverse-phase thin-layer chromatography. The isopropyl alcohol—water mixture (volume ratio 50: 50) was used as a mobile phase. Visualization with iodine vapour showed only one chromatographic zone with Rf = 0.66. This fact together with the determined values of density (d204 = 1.028 g/cm3) and refraction index (n20d = 1.5131) that were in accordance with the literature data testified the complete purification of the monomer. All other reagents used were of chemical or analytical purity grade and were purchased from REAKHIM, Russia [9].For the synthesis in homogeneous conditions was selected the complex solvent, in the capacity of which used a mixture of water, acetic acid and isopropyl alcohol (IPA). Synthesis of copolymers carried out at constant mixing in current of gaseous nitrogen. In the course of the experiment varied concentration of polymer, monomer, initiator and the temperature. For the copolymers extraction the solvent was evaporated at room temperature. Because in the presence of the KPS in the reaction mixture takes place as graft copolymerization of N-VCL onto CS, so homo polymerization of N-VCL. Therefore, the product of the reaction is a mixture of grafted copolymer and poly – N-VCL. For the separation of homopolymer and unreacted monomer N-VCL conducted extraction resulting mixture of isopropyl alcohol, not dissolved copolymer was separated and dried under vacuum up to a constant mass. Poly – N-VCL allocated by resedimentation from isopropyl alcohol to hexane with the subsequent drying to constant weights under vacuum. The fact of copolymer formation was confirmed by IR - spectroscopy, a method of gravimetry and elemental analysis. The composition of copolymer was calculated based on the content of total nitrogen, which was determined by the Dumas method The standard deviation from the arithmetic average for the samples with a nitrogen content exceeding 9% did not exceed 0.15%, in the content of nitrogen in copolymer less than 9% of the standard deviation does not exceed 0.1%. These results correspond to the accuracy of determining the composition ±8.2% and ±5.7%, respectively. The effectiveness of grafting (EG) of N-VCL in copolymers was determined on the basis of gravimetric data according to the formula:
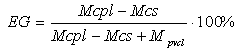 Where:Mcpl - the mass of grafted copolymerMcs - the mass of ChitosanMpvcl - the mass of poly-N-VCL
Where:Mcpl - the mass of grafted copolymerMcs - the mass of ChitosanMpvcl - the mass of poly-N-VCL3. Results and Discussion
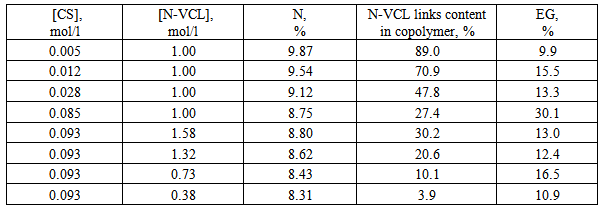
- Table 1 shows the dependence of the copolymer composition on concentration of CS and N-VCL in the reaction mixture. It is seen that the content of the N-VCL links in copolymer significantly depends on the ratio of CS:N-VCL (fig.1). The copolymers are enriched with N-VCL links at low concentrations of CS. An increase in the ratio CS:N-VCL leads to a rather sharp decrease of the content of the links of the N-VCL in the copolymer. Thus, by varying the concentration of polymer and N-VCL in the reaction mixture can significantly influence on the contents of the N-VCL in graft copolymer, synthesizing system in the widest range of grafting degrees. On the other hand, effectiveness of grafting (EG), which essentially characterizes the relationship between the reactions of homo - and copolymerization remains in the whole range of relations CS/N-VCL quite low. It indicated about prevalence of homo-polymerization N-VCL reaction compared with copolymerization. It should be noted that copolymers containing a large number of links N-VCL (more than 80%) dissolved in the water.
 | Figure 1. The dependence of the nitrogen content (1) in copolymer and content of N-VCL links in copolymer (2) on the molar ratio CS/N-VCL in the reaction mixture |
|
|
|
4. Conclusions
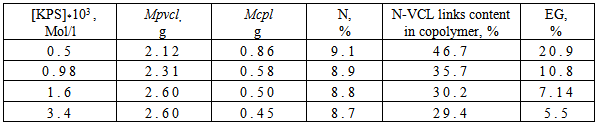
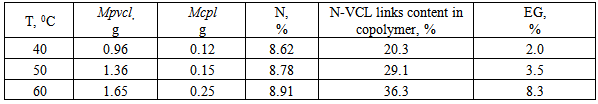
- CS, synthesized from chitin, selected from the pupae of the silkworm, modified by grafting N-VCL in the conditions of radical initiation in the presence of the KPS. By varying the concentration of CS and VC in the reaction mixture can be synthesized graft copolymers in a wide range of ratios of components and, accordingly, the extent of grafting. Copolymers, enriched with N-VCL links, obtained in excess of the monomer at a temperature of 60℃. The initiator concentration in a more significant impact on the copolymers structure, than on their composition. Copolymerization characterized by low values of the effectiveness of grafting, that is caused by dominance of the reaction N-VCL homo-polymerization over graft copolymerization.
References
| [1] | N. Sanoj Rejinold, K.P. Chennazhi, S.V. Nair, H. Tamura, R. Jayakumar. Carbohydrate Polymers. 83 (2011) 776. |
| [2] | Mani Prabaharan, Jamison. J. Grailer, Douglas A. Steeber, Shaoqin. Macromolecular bioscience 8 (2008) 843. |
| [3] | Mithilesh Yadav, Arpit Sand, Kunj Behari. International Journal of Biological Macromolecules. 50 (2012) 1306. |
| [4] | Dinesh Kumar Mishra, Jasaswini Tripathy, Abhishek Srivastava, Madan Mohan Mishra, Kunj Behari. Carbohydrate Polymers. 74 (2008) 632. |
| [5] | Abhishek Srivastava, Dinesh Kumar Mishra, Kunj Behari. Carbohydrate Polymer. 80 (2010) 790. |
| [6] | Jasaswini Tripathy, Dinesh Kumar Mishra, Mithilesh Yadav, Kunj Behari. Carbohydrate Polymers 79 (2010) 40. |
| [7] | V.O. Kudyshkin, R. Yu. Milusheva, A. M. Futoryanskaya, M. Yu. Yunusov, and S. Sh. Rashidova, Russian Journal of Applied Chemistry 80 (2007) 1750. |
| [8] | S. Sh. Rashidova, R. Yu. Milusheva. Hitin I hitozan Bombyx mori: Sintez, svoysva I primenenie. Tashkent: FAN, 2009. P. 246. |
| [9] | V.O. Kudyshkin, N.I. Bozorov, O.E .Sidorenko, I.N. Ruban, N.L. Voropaeva, G. Kogan, and S.Sh. Rashidova, Chem. Papers. 58 (2004) 286. |
| [10] | L.A. Nud'ga, V.A. Petrova, M.F. Lebedeva and G.A. Petropavlovskii, Russian Journal of Applied Chemistry 69 (1996) 1058. |
| [11] | L.A. Nud'ga, V.A. Petrova, N. V. Klishevich, L. S. Litvinova, A. Yu. Babenko and V. N. Shelegedin. Russian Journal of Applied Chemistry. 75 (2002) 1678. |
| [12] | Shih-Chang Hsu, Trong-Ming Don, Wen-Yen Chiu, Polym. Degrad. Stab. 75 (2002) 73. |
| [13] | E. N. Fedoseeva, Yu. D. Semchikov, and L. A. Smirnova, Polymer. Sci 48 B (2006) 295. |
| [14] | A. A. Kholmuminov, V.O. Kudyshkin, A. M. Futoryanskaya, O. B. Avazova, R. Yu. Milusheva, and S. Sh. Rashidova, Polymer. Sci 52 A (2010) 939. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML