-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2014; 4(2): 34-39
doi:10.5923/j.ijmc.20140402.03
Gmelina Arborea Bark Extracts as a Corrosion Inhibitor for Mild Steel in an Acidic Environment
Lebe A. Nnanna1, Kings O. Uchendu1, Francis O. Nwosu1, Uche Ihekoronye2, Eti P. Eti3
1Physics/Electronics Department, Abia State Polytechnic, Aba, Nigeria
2Civil Engineering Department, Abia State Polytechnic, Aba, Nigeria
3Mechanical Engineering Department, Abia State Polytechnic, Aba, Nigeria
Correspondence to: Lebe A. Nnanna, Physics/Electronics Department, Abia State Polytechnic, Aba, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
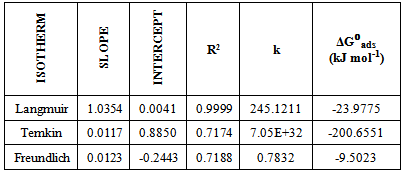
This paper describes the gravimetric analysis on the acidic corrosion of mild steel in the presence of different concentrations of Gmelina arborea bark extract at room temperature. G. arborea bark extract lowered corrosion rate of mild steel from about 0.6mm/yr to as low as 0.04mm/yr, which brought about very high inhibition efficiency values from 94% to an optimum value of about 96% after 40 hours of exposure. The mechanism of adsorption was investigated using the Langmuir, Temkin and Freundlich adsorption isotherms. A mixed adsorption mechanism (physisorption and chemisorption) was proposed. Adherence of the inhibitor on the metal surface was spontaneous given the negative values of the Gibb’s free energy of adsorption. Langmuir isotherm confirmed that physisorption occurred but the Temkin isotherm showed more of chemisorption occurrence than physisorption.
Keywords: Mild Steel, Corrosion inhibition, Gmelina arborea, Adsorption mechanism
Cite this paper: Lebe A. Nnanna, Kings O. Uchendu, Francis O. Nwosu, Uche Ihekoronye, Eti P. Eti, Gmelina Arborea Bark Extracts as a Corrosion Inhibitor for Mild Steel in an Acidic Environment, International Journal of Materials and Chemistry, Vol. 4 No. 2, 2014, pp. 34-39. doi: 10.5923/j.ijmc.20140402.03.
Article Outline
1. Introduction
- In pipeline, aircraft and general industries, acids such as HCl are used for oil well acidizing, prickling baths, removal of mill scales and sledges from metallic surfaces (Revie and Uhlig, 2008; Raja and Sethuraman, 2010; Li et al., 2012; Raja et al., 2013). When mild steel is used in these processes, it suffers severe corrosion. For the prevention of acid corrosion of mild steel, addition of corrosion inhibitors can be used, to protect the metal surface from aggressive acid attack effectively (Raja and Sethuraman, 2010; Raja et al., 2013). Most of the corrosion inhibitors are organic compounds, having hetereoatoms like nitrogen, sulfur, and oxygen, has been investigated as good corrosion inhibitors (Popova et al., 2004; Ahamad & Quraishi, 2009; Shukla & Quraishi, 2009; Singh & Quraishi, 2009; Hosseini & Azimi, 2009; Ahamad et al., 2010; Singh et al., 2010). Nevertheless, most of these compounds are not only expensive but also toxic to living being and environmental unfriendly. Due to the toxicity of some corrosion inhibitors, there has been increasing search for green corrosion inhibitors (Muthukrishnan et al, 2013). Most of the natural inhibitors are environmentally friendly, non-toxic, biodegradable, inexpensive, and readily available in plenty (Oguzie et al., 2010; Lebrini et al., 2011; Okafor et al., 2008; El-Etre, 2007; Lecante et al., 2011; Satapathy, 2009). Corrosion inhibition of leaf extracts of Occimum viridis, Telferia occidentails, Azadirachta indica, and Hibiscus sabdariffa, Sida acuta, Aspillia Africana, henna, Nauclea latifolia, Euphorbia falcata and more on mild steel in acidic solutions have been investigated (Oguzie, 2008; Edouket al., 2012; Mejeha et al., 2013; Hamdy and El-Glendy et al., 2013; Uwa et al., 2013; El Bribri et al., 2013). G. arborea is a fast growing tree, moderately adaptable and survives well on a wide range of soil types: acid soils, calcareous loams, and lateritic soils. It performs best on fresh, well-drained, fertile soils where rainfall annually varies from 1200 to 4500 mm (Kijkar, 2002). G. arborea have been reported to be used in case of hallucination, fever, dyspepsia, hyperdipsia, hemorrhoids, gastralgia, anasarca and in burning sensation (Rastogi & Mehrotra, 1990; Sarin, 1996; Chopra et al, 1999). G. arborea were found to contain alkaloids, carbohydrate, cardiac glycosides, tannis, phenolic compounds, saponins and flavonoids (Acharya et al., 2012; Nayak et al., 2012; Kulkarn & Veeranjaneyulu, 2013).The aim of this work is to investigate the corrosion of mild steel in 1.0 M Hydrochloric acid (HCl) by the leaf extract of Gmelina arborea as corrosion inhibitor by weight loss method. The adsorption parameters were calculated and discussed.
2. Materials and Methods
2.1. Mild Steel Preparation
- Mild steel specimens (C = 0.08 wt.%, Si = 0.05 wt.%, P = 1.00 wt.%, Cu = 0.02 wt.%, Pb = 0.02 wt.% and Fe = 98.83 wt.%) of dimensions of 20 x 20 x 1 mm were used for the gravimetric study. The surface preparation of mechanically polished specimens was carried out using different grades of emery paper and then degreased with acetone and air-dried.
2.2. Preparation of G. arborea Extract
- G. arborea barks were collected around Umungasi, Aba. The plant barks were cleaned, dried, ground, and soaked in distilled water. The crude extracts were boiled at 75℃ in reflux apparatus for 3 h, cooled, and filtered. The amount of ground bark material extracted into solution was quantified by comparing the weight of dried residue with initial weight of the dried bark material before extraction. From the respective stock solutions, inhibitor test solution was prepared in 5 concentrations ranged from 0.1 to 0.5 g/L.
2.3. Gravimetric Technique
- The polished and pre-weighed mild steel specimens of uniform size were suspended in 300 ml test solutions with and without the inhibitor at different concentrations for a period of 40 h. Then, the specimens were washed, dried and weighed. The mass-loss was calculated. From these data, inhibition efficiency (I%) was calculated using the following the equation:
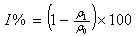 | (1) |
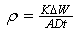 | (2) |
3. Results and Discussion
3.1. Gravimetric Studies
- The values of corrosion rates and inhibition efficiencies obtained from weight loss measurements for different concentrations of G. arborea in 1.0 M HCl after 40 h shown in Figures 1-3 below. From these charts, it is clear that corrosion rate decreased significantly with addition of the inhibitor and then decreased gradually with increasing inhibitor concentration, while inhibition efficiency increased with increasing inhibitor concentration, reached at a maximum value of 96.1%. G. arborea contain tannins, alkaloids and phenols which have fused benzene rings and O-heteroatoms in the ring. Due to the complex compounds, it is difficult to assign the inhibiting action to a particular constituent or group of constituents. The inhibitor molecules present in the extracts block the surface of mild steel via adsorption mechanism.
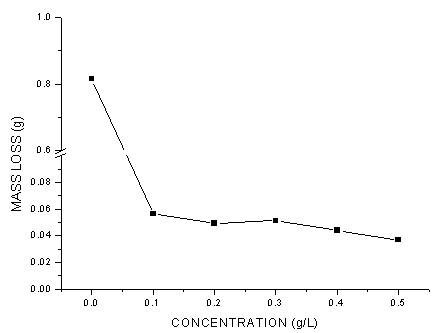 | Figure 1. Mass-loss of Mild Steel with and without the Gmelina arborea bark extract |
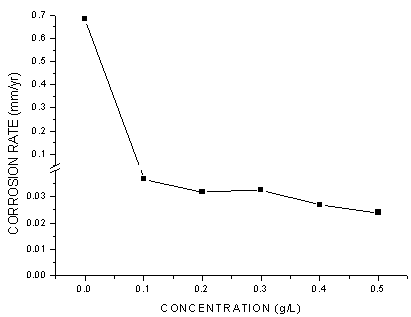 | Figure 2. Corrosion Rate of Mild Steel with and without the Gmelina arborea bark extract |
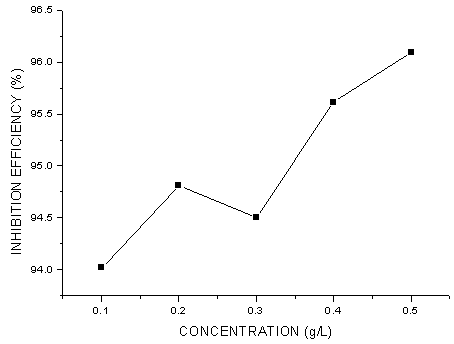 | Figure 3. Inhibition Efficiency of Gmelina arborea bark extract on Mild Steel Corrosion in 1.0 M HCl |
3.2. Adsorption Mechanism
- Generally, two modes of adsorption could be considered. The inhibition of the mild steel corrosion can be attributed to either the adsorption of G. arborea molecules or the formation of a layer of insoluble complex of the metal on the surface which acts as a barrier between the metal surface and the corrosive medium - physisorption.Secondly, the neutral G. arborea may be adsorbed on the metal surface via the chemisorption mechanism involving the displacement of water molecules from the metal surface and the sharing of electrons between oxygen atom and iron.Thus, Langmuir, Temkin and Freundlich approach were used to determine the adsorption mechanisms of the inhibition reaction. Langmuir and Freundlich isotherms have the potential to determine the physisorptions occurrence, while Temkin isotherm is used to examine for chemisorption.The consideration of the relationship between surface coverage and inhibitor concentration is expressed by Langmuir in Equation 3 and the constant K is the equilibrium constant which is employed in calculating the Gibb’s free energy shown in Equation 4 (Rajendran et al., 2000; Oguzie, 2005; Arab & Turkustuni, 2006; Ashassi-sorkhabi et al., 2006)
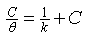 | (3) |
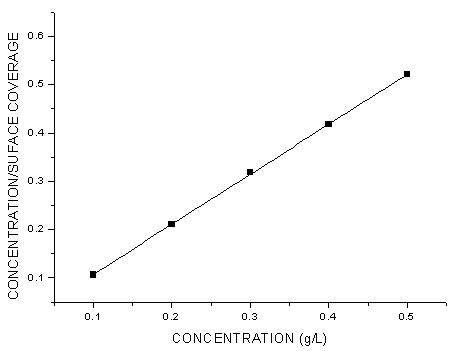 | Figure 4. Langmuir Isotherm Plot of the Corrosion of Mild Steel in the Presence of G. arborea Bark Extract |
|
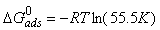 | (4) |
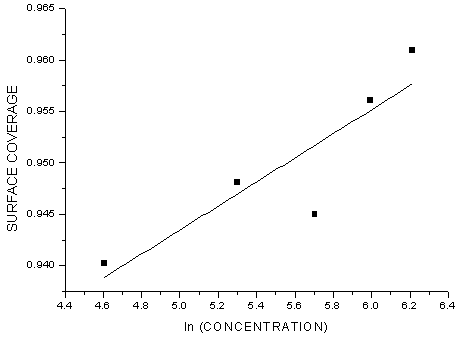 | Figure 5. Temkin Isotherm Plot of the Corrosion of Mild Steel in the Presence of G. arborea Bark Extract |
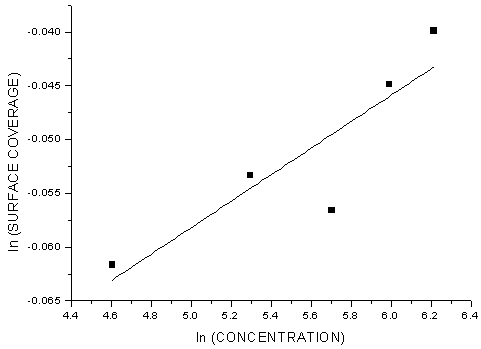 | Figure 6. Freunlich Isotherm Plot of the Corrosion of Mild Steel in the Presence of G. arborea Bark Extract |
4. Conclusions
- The present study shows that G. arborea bark inhibited the corrosion of mild steel in acidic medium of 1.0 M HCl. The inhibition efficiency at different concentrations recorded an optimum value of about 96.1%. The value of Gibbs free energy of adsorption indicates that Gmelina arborea leaf extract were physically and chemically adsorbed on the surface of the metal. The Langmuir and Temkin isotherms were obeyed which confirms a mixed adsorption mechanism given the spontaneity of the process. Thus, G. arborea bark extract can be used to inhibit mild steel corrosion in 1.0 M HCl.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML