-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2014; 4(1): 9-13
doi:10.5923/j.ijmc.20140401.02
Investigation of Corrosion Resistance of Polystyrene as an Inhibitor in Hydrochloric and Tetra-oxo Sulphate VI Acids
M. D. Shittu, J. O. Olawale, M. O. Adeoye, K. M. Oluwasegun, K. M. Adebayo, O. O. Ige
Department of Materials Science and Engineering, Obafemi Awolowo University, Ile-Ife, Nigeria
Correspondence to: M. D. Shittu, Department of Materials Science and Engineering, Obafemi Awolowo University, Ile-Ife, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This work investigated the effect of polystyrene on the corrosive behaviour of hydrochloric (HCl) and tetraoxo sulphate VI (H2SO4) acids on mild steel using weight loss technique. Cold rolled mild steel sample with chemical composition C: 0.21%, Mn: 0.593%, Si: 0.044, S: 0.032% and Fe: 98.4% was used. Five different concentrations of the polystyrene were used in 200ml solution of the acids. The corosion rate and inhibition efficiencies of polystyrene as a corrosion inhibitor were determined at different concentrations in HCl and H2SO4 as it was varied from 1 ml to 5 ml. It was observed that corrosion of the mild steel reduced as the concentration of polystyrene increased but decreased with increase in time. From the work also, one can deduce that polystyrene is a good inhibitor going by its efficiencies in HCl and H2SO4 with up to about 90% efficiency for HCl and 84% efficiency for H2SO4. It was therefore concluded that introduction of polystyrene into acidic medium where mild steel is used can be an economic way of corrosion prevention.
Keywords: Corrosion Inhibitor, Polystyrene, Mild Steel, Adsorption
Cite this paper: M. D. Shittu, J. O. Olawale, M. O. Adeoye, K. M. Oluwasegun, K. M. Adebayo, O. O. Ige, Investigation of Corrosion Resistance of Polystyrene as an Inhibitor in Hydrochloric and Tetra-oxo Sulphate VI Acids, International Journal of Materials and Chemistry, Vol. 4 No. 1, 2014, pp. 9-13. doi: 10.5923/j.ijmc.20140401.02.
Article Outline
1. Introduction
- Corrosion is a surface phenomenon, which attack and destroy materials by reaction with their environments electrochemically (Callister, 1997). Corrosion of metals has remains a world-wide problem as it destroys, most very often catastrophically, engineering infrastructures, since time immemorial. It occurs in various forms and it is promoted by variety of causes; all related to process and operation conditions. It is a continuous problem that can lead to the contamination of process streams which lead to poor production quality and unscheduled equipment shut downs. It can also lead to reduced production, high maintenance cost and equipment replacement (Nathan, 1973 and Sastri, 1998).The effect of corrosion can best be appreciated by the annual cost of its prevention, the direct and indirect value of which, according to Wall Street Journal (1981) is put at over hundreds of millions of US dollars all over the world. It was also predicted that costs of corrosion will escalate substantially during the next decade because of worldwide shortages of construction materials, higher energy costs, aggressive corrosion environments and other factors (Fontana, 1986 and Uhlig, 1971). Due to the problems arising from corrosion, several methods of corrosion prevention and protection of engineering infrastructures have evolved over the years. These include the use of protective coatings, proper design (Ross, 1985), cathodic and anodic protection, proper material selection and altering the environment (use of inhibitor). However, if it is well understood and controlled, corrosion can be used in a powerful and constructive manner for electrochemical production of fine patterns on metals as well as on semiconductor surfaces. This includes electrochemical machining (down to micro-scale or even nano-scale (Olakunle and Ajayi, 2005). Corrosion inhibition is a surface process which involves the adsorption of the inhibiting compounds on the surface of the metal. The definition of inhibitor favoured by the National Association of Corrosion Engineers (NACE, 1984 & 2002) is: a chemical substance that, when added in a small concentration to an environment, effectively decreases the corrosion rate.Selection of an appropriate corrosion inhibitor, inhibitor combination or package is an exceptionally cost effective and materials saving measure in various industries. Current trends and novel approaches in corrosion inhibitors are reviewed.. Recent developments in the inhibition technology are discussed in accordance with the areas of application of inhibitors (Maayta and Al-Rawashdeh, 2004).Through the years, sophisticated corrosion inhibitor test methods, typically designed to reproduce the most extreme conditions in a system, have been employed to improve inhibitor capabilities. New and better corrosion inhibitors have been developed as a result of their performance in elaborate laboratory apparatus, yet many have not achieved comparable performance in the field. The inability to transfer inhibitor performance from the laboratory to the field remains a challenge today. However, correlation of laboratory and field performance may be possible once key factors involved in inhibitor chemistry and corrosion theory are considered.Polymers are ideal for many industrial applications because of their desirable inert properties (Umoren et al., 2009, Umoren and Solomon, 2010; Ashassi and Ghalebsaz, 2005).Past works have reviewed the use of polymeric materials with additives as inhibitors for corrosion prevention. The uses of polyanilline and polyphosphate as inhibitors have been studied. Polymers such as alkyl acryl amide and polyphenylene either in conjunction with hexavalent chromium have also been used for environmental reasons. More also, there are several polymers available for studies and therefore the need to study their viability to serve as inhibitors. Abiola and Oforka (2004) also ascertained that altering the environments provide a versatile means of reducing corrosion. A survey of several works revealed the applicability of organic compounds as corrosion inhibitors for aluminium in acidic media (Abdallah et al; 2012). A large number of organic compounds, particularly those containing nitrogen, oxygen and sulphur in a conjugated system, are known to be applied as inhibitors to control acid corrosion of iron and steel. According to Schweitzer (2007), the inhibition process has been shown to occur via inhibitor adsorption isotherm and the efficiency of the inhibitors strongly depend on the structure and the chemical characteristics of the adsorbed inhibitor layer formed under particular experimental conditions. Mansfield (1987) reported the physicochemical aspects of metal corrosion in aqueous solutions and the main mechanisms involved in the formation of protective layers on metals by inhibitors.This present work intended to provide another means of solving corrosion problem by the use of polystyrene powder as inhibitor on mild steel or other form of steel, which may be exposed to unfriendly environment, most especially acidic medium.
2. Materials and Methods
- The mild steel used in this work was analysed to be of the following chemical composition C: 0.21%, Mn: 0.593%, Si: 0.044%, S: 0.032% and Fe: 98.4%. It was a cold rolled mild steel. The choice of this sample was predicated on the fact that it is about the most widely used steel material due to its low cost, availability and their good fabrication qualities (working and welding characteristics). The material found its use in reaction vessels such as cooling tower tanks, boilers, pipelines, sections for use in bridges etc. (Davies and Oelmann, 1983).Other materials used include: − analytical grade hydrochloric acid (HCl) and tetraoxosulphate VI (H2SO4) acids which are frequently used in industries alongside the mild steel components.− Ethanol and acetone (for washing of the coupons during corrosion monitoring)− Desiccators and Mettler analytical weighing balance The as-received mild steel samples were machined into cylindrical test specimens of about 10 mm diameter and length of 30 mm using lathe machine. The specimens were slightly ground and polished. They were then sequentially degreased with ethanol, rinsed in distilled water and dried in acetone. The specimens were then stored in desiccators.Concentrated HCl acid of density 1.18 g/ml, percentage purity of 38 and molar mass of 36.47 g/mol was prepared into 200 ml solution. Similarly, H2SO4 of density 1.84 g/ml and molar mass 98.08 g/mol was prepared. Different concentrations of polymer solutions were also prepared by dissolving 1 ml to 5 ml polystyrene in 200 ml of the solution separately.Three of the steel specimens earlier kept in desiccators were weighed and fully suspended in each of the 200 ml HCl and 200 ml H2SO4 (which act as control experiments). The remaining steel specimens were equally weighed and a set of three suspended in acid with different concentration of the polystyrene solutions at standard room temperature. The weight loss for each of the specimen was determined in 48 hours progressively for 240 hours and this was used to determine the corrosion rate in gcm-2h-1.The total surface area of each specimen (being cylindrical) was determined using the expression
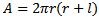 | (1) |
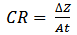 | (2) |
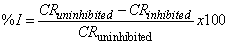 | (3) |
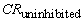 = corrosion rate of the uninhibited system and
= corrosion rate of the uninhibited system and 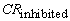 = corrosion rate of the inhibited system.
= corrosion rate of the inhibited system.3. Results and Discussion
3.1. Results
- The results of weight loss were used to calculate, in accordance with equation (2) above, the corrosion rate, in the HCl with and without inhibitor are shown in Fig.1 while Fig. 2 similarly shows the corrosion rate in uninhibited and inhibited H2SO4. Fig.3 depicts the relationship between the inhibition concentration and inhibitor efficiency (%I) in both HCl and H2SO4.
3.2. Discussion
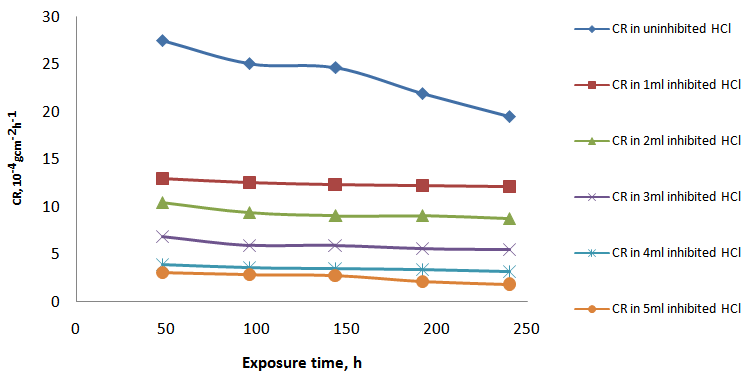
- Visual observation of the coupons under corrosion testing showed that there was formation of thin film on the metal surface and the film thickness increases as the polystyrene concentration increases. Figure 1 and Figure 2 showed the corrosion rate in presence of 200ml HCl and H2SO4 solution at varying concentration of polystyrene respectively. From the two figures (Fig. 1 and Fig. 2), it was observed that the corrosion rate decreased with exposure time and the rate of decrease increases with increase in polystyrene concentration. This may be ascribed to a adsoption of the organic (polymer) molecules on the corroding surface of the mild steel as deduced by Morad (1999) which was also corroborated by the work of Paul et al (2011) where drag reducing polymer was used and observed that there was percentage decrease in the rate of corrosion with increase in polymer concentration. Fig. 2 shows a similar trend to Fig. 1 but it was observed that the weight loss and, of course, corrosion rate in HCl system is higher than its corresponding H2SO4 system. This could be due to the fact that HCl is considered a stronger acid than H2SO4 as indicated by Hill and Holman (1979). Also, Fig.1 showed that corrosion rate increases for some time and then reaches the relatively stable value in HCl whereas the corrosion rate keeps increasing during all the exposure time for H2SO4 (Fig.2). This is likely due to the time of passivity of the two acids that differs as a result of their difference in corrosivity. From Fig. 3, it could be observed that the efficiency of polystyrene as an inhibitor increase with increase in its concentration in the two acids. In HCl, the efficiency rose from 47% with 1ml of the inhibitor to 200ml of HCl solution to 90% as the concentration of the polystyrene rose to 5ml to 200ml HCl solution. Similarly, in H2SO4, the range of inhibitor efficiency (%I) is from 38% to 84% for 1ml and 5ml respectively of the inhibitor in 200ml H2SO4. This may be attributed to the thickness of the layer formed on the metal by the polymer material which may be preventing the discharge of
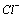 and
and 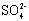 and quick dissolution of metal ions. The increase in inhibition efficiency (%I) was found to be relatively higher in HCl than in H2SO4. The cause for this difference could also be linked with the reason advanced by Hill and Holman (1979) earlier (Ashassi and Ghalebsaz, 2005).
and quick dissolution of metal ions. The increase in inhibition efficiency (%I) was found to be relatively higher in HCl than in H2SO4. The cause for this difference could also be linked with the reason advanced by Hill and Holman (1979) earlier (Ashassi and Ghalebsaz, 2005).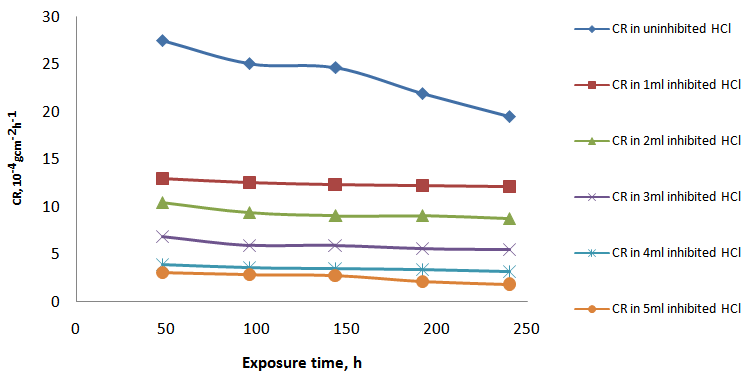 | Figure 1. Curves of corrosion rate against exposure time in uninhibited and inhibited HCl |
 | Figure 2. Curves of corrosion rate against exposure time in uninhibited and inhibited H2SO4 |
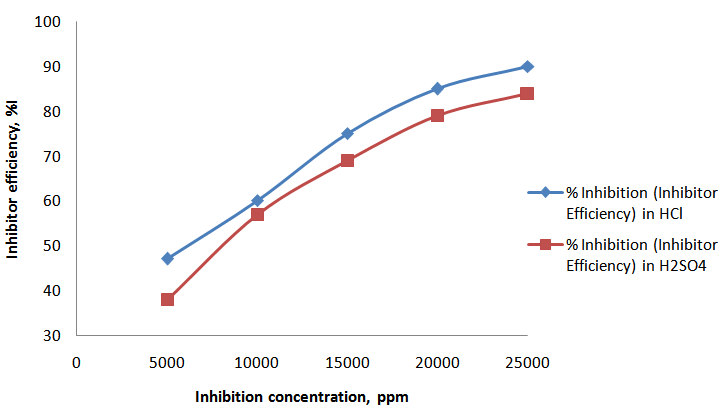 | Figure 3. Curves of inhibition concentration against inhibitor efficiency in HCl and H2SO4 |
4. Conclusions
- This investigation has revealed that an addition of polystyrene in small quantity reduced the rate of corrosion on mild steel in aqueous solutions of both HCl and H2SO4 as the rate of corrosion decreased with increase in polystyrene concentration in the two acids. Also, the high magnitude of the inhibitor efficiencies of 90% and 84% respectively for both HCl and H2SO4 solutions is an indication to conclude that polystyrene is a good corrosion inhibitor for mild steels in acidic environments. Therefore, corrosion of mild steels commonly used in chemical industries and other acidic environments can be minimized by very simple and inexpensive means as the use of polystyrene inhibitor.
ACKNOWLEDGEMENTS
- The assistance and contributions of the academics, administrative and the technical staff of the Department of Materials Science and Engineering of Obafemi Awolowo University, Ile-Ife, Nigeria are greatly appreciated for this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML