-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2013; 3(5): 106-111
doi:10.5923/j.ijmc.20130305.04
Synthesis, Characterization and Magnetic Properties of γ-irradiated and Unirradiated Magnetite Nanopowders
M. Khairy
Chemistry Department, Faculty of Science, Benha University, Benha, Egypt
Correspondence to: M. Khairy, Chemistry Department, Faculty of Science, Benha University, Benha, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
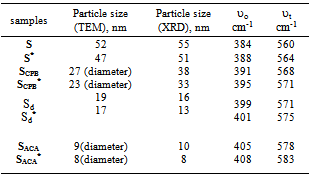
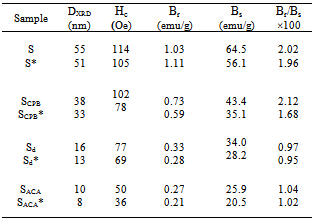
Nano-sized magnetite (Fe3O4) nanoparticles were prepared using dry and wet chemical methods in presence of surfactants as capping agents. The samples were characterized by X-ray diffraction, FT-IR, thermal analysis (DTA and TG), Electron microscopy (SEM) as well as (TEM), dynamic laser scattering analyzer (DLS) and vibrating sample magnetometer (VSM) techniques. The X-ray diffraction pattern show cubic spinel crystal structure for all samples. Particle size in the range of 8 - 55 nm is obtained. The effect of preparation method and γ- irradiation process on the magnetic properties of prepared samples was studied and discussed. All samples show soft-magnetic behavior with much lower coercivity and much higher saturation magnetization. The coercive force (Hc), saturation magnetization (Bs), remanent induction (Br) and the ratio of remanent induction to saturation magnetization (Br/Bs) are found to be size and shape dependent. The saturation magnetization value lies between 20.5 and 64.5 emu/g. The magnetic properties are explained by electron hopping mechanism between Fe2+ and Fe3+ -ions. The use of magnetite nanoparticles in preparation of ferrofluid was investigated. The ferrofluid stability increases with decreasing the particle size.
Keywords: Nanostructures, Magnetite, VSM, Gamma Irradiation, Magnetic Properties
Cite this paper: M. Khairy, Synthesis, Characterization and Magnetic Properties of γ-irradiated and Unirradiated Magnetite Nanopowders, International Journal of Materials and Chemistry, Vol. 3 No. 5, 2013, pp. 106-111. doi: 10.5923/j.ijmc.20130305.04.
Article Outline
1. Introduction
- Over the past few years, controlling the size and shape of metal oxides has attracted significant interest because the shape and size of these materials have significant influence on their chemical and physical properties[1-4]. Magnetite (Fe3O4), as one kind of important transition metal oxides, is of particular importance because of both its unique properties including magnetic properties, chemical stability, biocompatibility, and low toxicity and potential applications in magnetic recording and separation, catalyst, photocatalyst, pigments, ferrofluids, magnetic resonance imaging (MRI), drug delivery, etc.[5-8].There are several methods have been developed to synthesize Fe3O4 nanoparticles[9]. However, the shape controlled synthesis of Fe3O4 in the nanometer regime has limited success. The size and morphology of nanoparticles are two important characteristic influencing their electrical, optical and magnetic properties[10-13]. The difference between nano and bulk materials has immense theoretical and technological importance. The way nanoparticles are synthesized may determine their morphological uniformity and size distribution; these conditions become one of the key challenging issues in nanoscience and nanotechnology[13]. Most commonly, Fe3O4 nanoparticles are prepared via co-precipitating ferrous and ferric ions in aqueous solution[14]. However, the corresponding chemical reactions do occur very fast, just promptly on mixing reactants, and this makes it very difficult to control the crystallization process. As a consequence, the magnetic nanoparticles of the resulting iron oxides tend to exhibit relatively poor size uniformity and crystallinity, and this feature may limit their use in many technological applications.Ionizing radiation such as gamma ray has frequently been used in studies of the physical properties of crystalline solids[15]. Structural defects can be introduced by ionizing radiation. Severe disruption of the lattice of a crystalline solid is possible, with the formation of a large number of defects. In this context, in the present investigation, dry and wet chemical methods have been employed to synthesized Fe3O4 powders with an average particle size down to several nanometers. The XRD, FTIR, SEM and TEM techniques were used to characterize the structure, purity, and the size of the nanoparticles. The magnetic properties were evaluated by vibrating sample magnetometer (VSM) measurement. The magnetization curves of these nanocrystals have been tested and analyzed. The relationship between preparation methods and the magnetic properties of resulting materials identified via the above methods was discussed. The influence of gamma ray on the magnetic properties of the synthesized nanoparticles was examined. The investigated Fe3O4 nanoparticles were dispersed into aqueous citric acid to obtain nanofluids and their stabilities were tested.
2. Experimental
2.1. Materials
- Analytical pure ferrous nitrate (Fe(NO3)2·4H2O), Ferric nitrate (Fe(NO3)3·9H2O), ferrous oxalate dihydrate (FeC2O4.2H2O), ammonium hydroxide (NH4OH, 28–30% of ammonia), 1-adamantanecarboxylic acid (ACA, 99%), and cetylpyridinium bromide (CPB) were purchased from Aldrich and used for Fe3O4 synthesis.
2.2. Synthesis of Magnetite Nanoparticles
- The magnetite samples were prepared using both thermal decomposition and wet chemical methods in the presence and absence of surfactants as follows:(a) Wet chemical methodFe3O4 nanoparticles were synthesized according to the coprecipitation method. A typical preparation process is described as following: a definite weights of Fe(NO3)2·4H2O (21.4 g) and Fe(NO3)3·9H2O (28.9 g) were dissolved in distilled water in presence of 10 mmol surfactant (50 ml) with a nitrogen protection. After the solution had been bubbled with nitrogen for 30 min, 0.1 mol/L NH4OH solution was introduced slowly into the system to adjust the pH above 11. The system was continuously bubbled with nitrogen for 20 min to remove oxygen. The formed black precipitates were collected from the solution by centrifugation, washed with distilled water several times, and dried at 90℃ for 8 h. The composition of Fe3O4 was verified by KMnO4 titration method[16]. The results showed a ratio of Fe3+: Fe2+ agrees with the expected 2:1 ratio within 2%.The samples were denoted as S for the sample prepared without surfactant and as SCPB and SACA for the samples prepared using CPB and ACA surfactants, respectively.(b) Thermal decomposition dry methodFor the dry method, 10 g of ferrous oxalate dihydrate was thermally decomposed, at low oxygen partial pressure, for 6 h at T=773 K[17]. The obtained product was then milled in a planetary ball mill. The milling was performed in a closed container with a hardened-steel vial of 120 ml volume and 80 hardened-steel with a diameter of 10 mm at ambient temperature. The milling intensity was 200 rpm and the ball-to-powder weight ratio of 20:1 was chosen. The milling process was carried out for one hour. The sample is denoted as Sd. All dry magnetite samples were kept in desiccators over calcium chloride to avoid the oxidation to maghemite. The prepared samples were irradiated by γ-ray source using a 60Co gamma cell (60Co gamma cell 2000 Ci with a dose rate of 1.5 Gy/s (150 rad/s) at a temperature of 30℃. Each sample was subjected to a total final dose of 1x105 Gy (10 Mrad). The irradiated samples were distinguished from the unirradiated ones by adding an asterisk beside the symbol of the irradiated samples, e.g. Sd*.For preparing aqueous based ferrofluids, one gram from each one of γ-irradiated and unirradiated magnetite samples was suspended in 10 ml of 50 wt% aqueous citric acid and heated at 90oC for 90 minutes under vigorously mechanically stirring.
2.3. Instrumentation and Measurements
- Thermal analysis (DTA and TG), XRD, FT-IR, SEM and TEM techniques were employed to characterize thermal stability, structure and morphology of the investigated magnetite samples. The thermal analysis was recorded using Schimadzu DT-50 thermal analyzer with samples of about mg in a static air atmosphere and at heating rate 10 K/min. The thermograms showed thermal stability for all samples over a temperature range of 300 - 673 K. Phase identification of the prepared samples was carried out at room temperature by X-ray diffraction (XRD) patterns using a Philips diffractometer PW 1710 with Cu-Kα irradiation (λ=0.15405 nm). FT-IR spectra were performed by FT-IR spectrophotometer model Perkin-Elmer 599 using KBr pellet technique. Scanning electron microscopy (SEM) and Transmittion electron microscopy (TEM) were taken using electron microscope models JEM-5200 Joel and Joel 2010 respectively. The volume-average diameter and size distribution of Fe3O4 magnetic nanoparticles in ethanol were measured by dynamic light scattering (DLS) using Malvern HPPS5001 laser particle-size analyzer at 25℃. Magnetic measurements were measured at room temperature using a vibrating sample magnetometer (VSM; 9600-1 LDJ, USA) in a maximum applied field of 15 kOe. Sedimentation method was used to test the ferrofluid stability of the studied samples. The sedimentation under gravity was carried out as described by Thies-Weesie et al.[18]. Weighted amounts of the ferrofluids were poured into cylindrical glass tubes of 1 cm diameter and 15 cm length and carefully closed. The tubes were vertically immersed in a water bath, placed on a heavy marble table to minimize vibrations. The equipment was placed in a thermostatic room to keep the temperature of the sedimentation dispersions constant at 25.0(0.1℃).
3. Results and Discussion
3.1. Characterization of the samples
- X-ray diffraction patterns of the synthesized Fe3O4 samples are shown in Fig. 1. All the observed peaks agree well with the corresponding reported JCPDS file (19-629) for the magnetite spinel structure. The crystallites sizes, DXRD, were estimated by using the Scherrer's equation[19], based on (311) peak: DXRD = 0.9 λ / β cosθ where λ is the X-ray wave length, θ the Bragg’s angle and β is the pure full width of the diffraction line at half of the maximum intensity. DXRD values obtained lie in the range of 8 - 55 nm, Table 1. The irradiated samples also exhibit the same crystalline phase with crystallite sizes in the same order with that found for unirradiated ones.
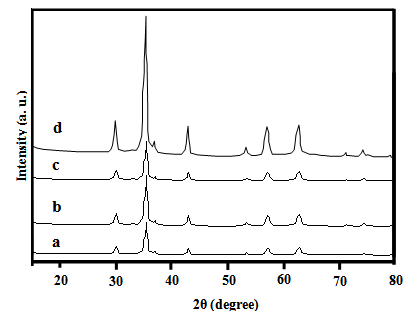 | Figure 1. XRD patterns of Fe3O4 samples: a)S b) Sd c) SCPB d) SACA |
|
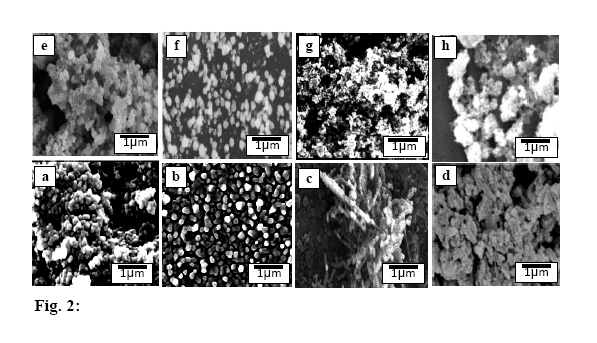 | Figure 2. SEM micrographs of: a)S b) Sd c) SCPB d) SACA e)S* f) Sd* g) SCPB* h) SACA* |
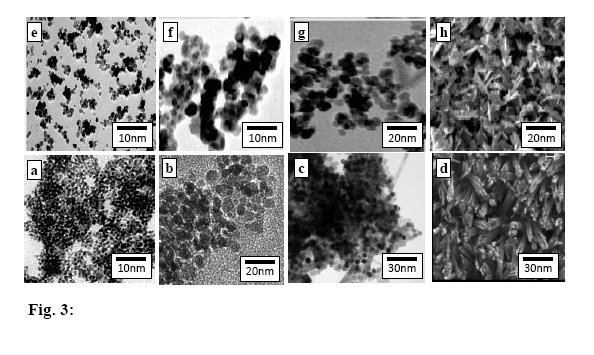 | Figure 3. TEM micrographs of: a)S b) Sd c) SCPB d) SACA e)S* f) Sd* g) SCPB* h) SACA* |
3.2. Magnetic Properties
- The basic property of any magnetic material is the relation between flux density and field strength. i.e. the B-H loop. For our samples, hysteresis loops with a normal (S-shape) type are observed at room temperature, Fig. 4 (a, b). The size and shape of the hysteresis curve are of considerable practical importance. The area within a loop represents a magnetic energy loss[22, 23]. This energy loss is defined as heat that is gratitude within the magnetic specimen and is capable of rising the temperature. The areas obtained for all samples are found to be small, and the loops are thin and narrow which is a specific criteria for soft ferrite. There is almost immeasurable coercivity at room temperature indicating that the prepared Fe3O4 samples are super paramagnetic. This super paramagnetic behavior means that the thermal energy can overcome the anisotropy energy barrier of a single Fe3O4 nano particle, and the coercivity and remanence of Fe3O4 nano particles are zero in the absence of external field. Saturation magnetic flux density (Bs), remnant magnetic flux density (Br) and the ratio of remanent induction to saturation magnetization (Br/Bs) were determined from B-H loops and listed in Table 2. From which it can be seen that the magnetic properties are size dependent. The coercivity Hc increases with increasing the crystallite size.The saturation magnetization Bs ranges from 25.9 - 64.5 emu/g which are in all cases far from the reported values for bulk Fe3O4 (Bs= 92 emu/g)[22]. This decrease obtained in saturation magnetization value Bs can be understood on the basis of the increase occurring in the magnetically dead layer thickness (nonmagnetic or weakly magnetic interfaces) with the decreasing the crystallite size. With decreasing the particle size, the surface to volume ratio increases and in turn the magnetically dead layer fraction increases. Moreover, the magnetic spins of the surface molecules of nano particles are disordered due to the incomplete coordination, which may also be a reason for lower saturation magnetization. Generally, it can be said that the total magnetization of the sample decreases with decreasing particle size due to the increase in the magnetic integral[23-25] and ultimately reaches the super paramagnetic state, when each particle acts as a big spin with suppressed exchange interaction between particles. These might be the reasons for the magnetic moment quenching and consequently for the decrease in the saturation magnetization.
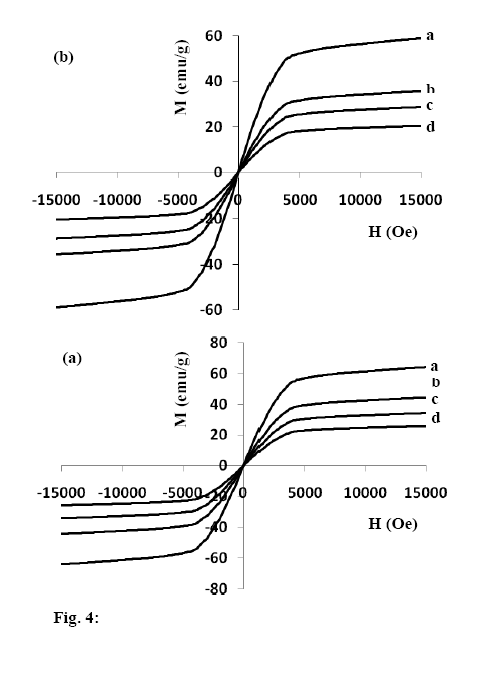 | Figure 4a. Hysteresis curves at room temperature for unirradiated samples, a)S b) SCPB c) Sd d) SACA b. Hysteresis curves at room temperature for irradiated samples, a)S* b) SCPB * c) Sd * d) SACA * |
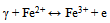 The obtained results may be also understood on the basis of crystal structure of Fe3O4. Magnetite (Fe3O4) is a ferrimagnetic iron oxide having cubic inverse spinel structure with oxygen anions forming an FCC closed packing and iron (cations) located at the interstitial tetrahedral sites and octahedral sites[22]. The electron can hop between Fe2+ and Fe3+ ions in the octahedral sites at room temperature imparting half metallic property to magnetite. The magnetic moment of the unit cell comes only from Fe2+ ions with a magnetic moment of 4μB[27]. Therefore, the decrease in Fe2+/Fe3+ ratio after irradiation process causes the decrease observed in Bs values for irradiated samples.
The obtained results may be also understood on the basis of crystal structure of Fe3O4. Magnetite (Fe3O4) is a ferrimagnetic iron oxide having cubic inverse spinel structure with oxygen anions forming an FCC closed packing and iron (cations) located at the interstitial tetrahedral sites and octahedral sites[22]. The electron can hop between Fe2+ and Fe3+ ions in the octahedral sites at room temperature imparting half metallic property to magnetite. The magnetic moment of the unit cell comes only from Fe2+ ions with a magnetic moment of 4μB[27]. Therefore, the decrease in Fe2+/Fe3+ ratio after irradiation process causes the decrease observed in Bs values for irradiated samples.
|
3.3. Aqueous Ferro Fluid
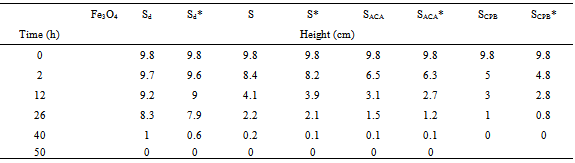
- A ferrofluid is a colloidal suspension of suitably coated magnetite particles in a liquid medium having unusual properties due to the simultaneous fluid mechanic effects and magnetic effects. Its wide spread applications are in the form of seals to protect high speed CD drives, as rotary shaft seals, for improving performance of audio speakers, in oscillation damping and position sensing[28], etc. A stable aqueous ferrofluid for our samples was prepared by dissolving the prepared nano particles in citric acid (used as dispersant) to form a stable aqueous dispersed magnetite particles, and simultaneously provide functional groups on the particle surface that can be used for further surface derivatization. The behavior of ferrofluids is mainly determined by their magnetic properties. Usually, they show superparamagnetic behavior considering each particle as a thermally agitated permanent magnet in the carrier liquid. In the presence of a magnetic field, H, the magnetic moment (μ) of the particles will try to align with the magnetic field direction leading to a macroscopic magnetization of the liquid. The magnetization, M, of the liquid behaves as the magnetization of a paramagnetic system.The stability of the ferrofluids prepared under the same conditions is found to depend on each of the particle size and morphology, Table 3. The samples with spherical particles (S and Sd) show higher stability than that of the other samples. Moreover, for the same morphology, the stability is found to increase with decreasing the particle size. The γ- irradiated samples show less stability than that of unirradiated ones. This is attributed to the smaller magnetic values of the irradiated specimens as compared with that of unirradiated ones.
|
4. Conclusions
- The results show that Fe3O4 nanoparticles can be produced in the sizes range from 8 to 55 nm by dry and wet chemical methods. The sizes and the morphologies of magnetite particles are affected by the operational parameters. The magnetic properties of nanosized ferrites show an apparent superparamagnetic behavior and the magnetization is affected by γ- irradiation process. The magnetite particles have saturation magnetization values (Bs) ranging between 20.5 and 64.5 emu/g, which are far from the reported values for bulk Fe3O4 (Bs = 92 emu/g). The magnetic parameters (Bs, Br and Hc) decrease with decreasing the particle size due to the existence of spin canting. Stable suspensions of magnetite nanoparticles in water (water-based ferrofluids) are prepared using citric acid as a dispersant. The stability of ferrofluids increases with decreasing the particle size of magnetite.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML