Andrianainarivelo Mahandrimanana, Ranaivoson Joseph
University of Antananarivo, Faculty of Science, Department of Inorganic Chemistry and Physical Chemistry
Correspondence to: Andrianainarivelo Mahandrimanana, University of Antananarivo, Faculty of Science, Department of Inorganic Chemistry and Physical Chemistry.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The aim of this work is to highlight the physicochemical properties of three various types of clay soils respectively collected at Bevalala (sample 1), Arivonimamo (sample 2) and Antsirabe (sample 3). The analyses obtained by x-ray fluorescence showed the variation of the composition of the major elements in each sample in particular the rates of SiO2 (47.6%, 41.2%, 52.5%), of Al2O3 (16%, 23%, 25%), of MgO (0.52%, 0.39%, 1.9%) and of Fe2O3 (8.3%, 4.7%, 6.4%) at 500°C.. The study of the plasticity of these samples (Ip1= 29.33, Ip2=14.5, Ip3= 24.16) which are intended for the manufacture of pot, of muds or of bricks allowed to highlight that the clay soil is more plastic than its plasticity index is important. The addition of CaCO3 and the heating of the sample at 500°C during five hours make it possible to increase the concentration in CaO.
Keywords:
Clay, X-ray Fluorescence Analysis, Plasticity
Cite this paper: Andrianainarivelo Mahandrimanana, Ranaivoson Joseph, Physico-Chemical Analysis for Differents Types of Clays Soils in the Areas of Analamanga, Itasy and Vakinankaratra, International Journal of Materials and Chemistry, Vol. 3 No. 5, 2013, pp. 99-105. doi: 10.5923/j.ijmc.20130305.03.
1. Introduction
In Antananarivo,yellow, gray or white clays, with different thickness, under a weak covering, offer reserves very limited on the industrial sphere because of presence of rice plantations on the surface. Only many artisanal exploitations for brickyard developed, of restricted extension.These clays, of complex composition: kaolinite, halloysite (hydrated kaolinite, beidellite, nontronite, cook red. Their reserves (not cubed) appear important but are partly obliterated by the presence of cultures on the surface. An exploitation is designed to feed the cement factory of Antsirabe.In the coastal area of Madagascar, the soil clays are not useful to build houses because there are a lot of sand. High-plasticity clays occur in many areas of Madagascar especially in the middle and often offer the most economical material alternative for construction of highway embankments. This study shows that in the three areas which we have chosen, the soils contain very important content of clays.The clays minerals[1] have several physical properties and chemical which are: their faculty to aspire water especially when they dry. They can also reject this water under the action of heat (phenomenon of hydration and dehydration) [2]. When they are mixed with water, one can obtain from it a paste intended for the ceramic manufacturing of product as the bricks and the tiles. The clay fraction is used because its plasticity is easy to calculate by the method of Casagrande. Obtaining a good paste is especially subordinate to this plasticity which depends on the chemical composition of clay. Then, we will study the plasticity of the clay soil according to the method of Casagrande. For the chemical properties of the grounds, we will see the chemical composition by the method of spectrometry of Fluorescence to X-rays (FRX).We will approach a particular study of the clay soils coming from the area of Bevalala, Arivonimamo and the area of the thermal spring of Antsirabe.
2. Theoritical Background
In general, the properties of the grounds are related to those of the minerals which constitute them.
2.1. Chemical Properties
2.1.1. Structure of the Clay
The clay minerals are presented under the aspect of the particles, crystallites or layers of very small sizes.
2.1.2. Ionic exchanges Property[3]
One of the most known properties of clays is their aptitude to fix some anions and especially the exchangeable cations at other cations and anions. The cations most easily exchangeable in clay materials are: Ca2+, Mg2+, H+, K+, NH4+, Na+ and the anions most easily, exchangeable are: SO42-, Cl-, PO43- and NO3. One can write for example an exchange of the cations:  Here a balance was established but if one systematically renews the solution with balance (by a new solution with balance) by a new solution NaCl, one obtains after several treatments a clay – Na and the number of equivalence of fixed Na+ is equal to that of moved Ca2+. This balance also depends on the concentrations of the exchangeable ions
Here a balance was established but if one systematically renews the solution with balance (by a new solution with balance) by a new solution NaCl, one obtains after several treatments a clay – Na and the number of equivalence of fixed Na+ is equal to that of moved Ca2+. This balance also depends on the concentrations of the exchangeable ions 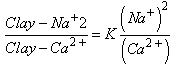 With K= the constant of balance If P is the power of absorption one a:
With K= the constant of balance If P is the power of absorption one a: 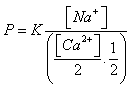 With[Ca2+] and[Na+] are expressed in equivalent grams.During the fixing of the Na+ ions, one can also define solutions used, the capacity of exchange of sodium (sodium adsorption ratio) noted:
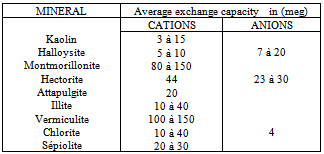
With[Ca2+] and[Na+] are expressed in equivalent grams.During the fixing of the Na+ ions, one can also define solutions used, the capacity of exchange of sodium (sodium adsorption ratio) noted: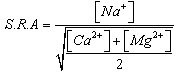 S.A.R is expressed in milliequivalents- gram for clay 100g. Values of the capacities of exchange of the cations and anions are given in the following table:
S.A.R is expressed in milliequivalents- gram for clay 100g. Values of the capacities of exchange of the cations and anions are given in the following table:Table 1. Value of exchange capacity of cations and anions[4]
 |
| |
|
2.1.3. Colloidal Property of the Clay Matter2
According to the colloidal property of the clay matter, the constituent micelles of a clay paste can slip the ones on the others. This property is supported by the presence of free water in the paste which plays to some extent a part of lubricant allowing the deformation and thus returning plasticity.In a perfectly purified clay i.e. not containing those ions H+ and OH-, we find the colloidal property of clay. Let us suppose that the load of the clay matter is due to the dissociation of clay micelle in micro anion (clay-OH) - and H+ or by adsorption of ions OH- of the aqueous medium on the side breaks of the crystal. The positively charged H+ ions revolve around micelles and are attracted by the negative charge of the surface of the core.
2.2. Physical Properties
2.2.1. Plastic Property of Clay
The plastic clay soils are very resistant to heat and are composed of fine particles of kaolin, quartz, illite and mica. One especially employs them in the manufacturing of pottery, dishes, tiles for walls, medical parts[5,6] (i.e.bathrooms). They are also used as agent of filling in rubbers, the plastics, paintings and the adhesives. B.2 Classification of a ground following its degree of plasticity With the index of plasticity, one can classify a soil according to his degree of plasticity.Table 2. degree of soil plasticity
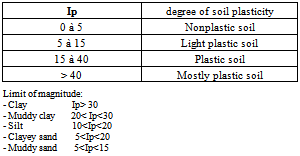 |
| |
|
3. Methods and Experiments
3.1. Determination of the Plasticity of Clay
We determine in experiments the plasticity of clays with the box of Casagrande. The limits of Atterberg make it possible to evaluate the plasticity of a clay soil and in particular the water content in the grounds. Indeed, water has a capital influence on the behavior of the clay soils and their cohesion. Casagrande began again and developed standardized tests making it possible to determine the water contents for which the transition is carried outTo determine Wl according to the formula: 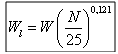 Wl: liquid limit NR: Many blows To calculate Wp (plastic limit). We take a sample of ground and one lets it dry (A). We add a little water and we rolls on a surface punt, a small glass plate for example. We try to form wire a 3 mm thickness and 10 cm length, without it breaking (B). We repeat this experiment by adding each time a little water until you can form a wire. The water content will correspond then to the plastic limit and could be expressed as a percentage weight of the sample.
Wl: liquid limit NR: Many blows To calculate Wp (plastic limit). We take a sample of ground and one lets it dry (A). We add a little water and we rolls on a surface punt, a small glass plate for example. We try to form wire a 3 mm thickness and 10 cm length, without it breaking (B). We repeat this experiment by adding each time a little water until you can form a wire. The water content will correspond then to the plastic limit and could be expressed as a percentage weight of the sample.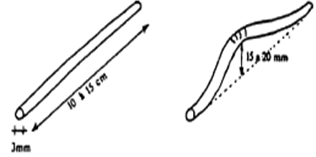 | Figure 1. test of plasticity |
Research of the limit of plasticity Ip: The calculation of the index of plasticity IP is determined by the formula: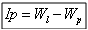 Wl and Wp being liquid limit and plastic limit
Wl and Wp being liquid limit and plastic limit
3.2. X-ray Fluorescence Spectrometry
The fluorescence-X spectrometry is a chemical method of analysis using a physical property of the matter, the fluorescence of X-rays. When one bombards matter with X rays, the matter emits energy in the form. The spectrum of the X-rays emitted by the matter is characteristic of the composition of the sample by analyzing this spectrum, we can determine the elementary composition from it and the mass concentrations in elements. We use this method to analyze our samples.
4. Results and Discussion
4.1. Results
4.1.1. Determination of the Plastic limit of the Samples
The aim of this study is to determine the plasticity of the samples in Bevalala, Antsirabe and Arivonimamo. We use the box of Casagrande to determine the different plastic limits of those samples.Ø Sample of Bevalala1. Liquid limit Wl:Table 3. Determination of the liquid limit of the sample of Bevalala
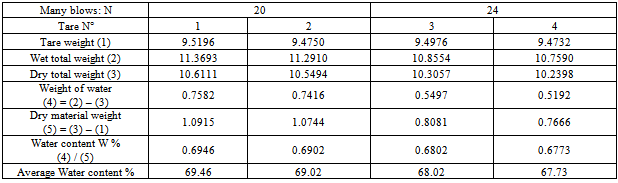 |
| |
|
2. Plasticity limit WP:Table 4. To Determine plasticity limit of the sample of Bevalala
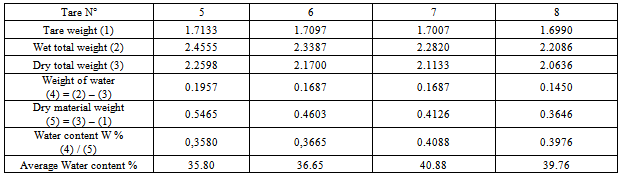 |
| |
|
3. Plasticity index Ip:Liquid limit: W l= 67.59Plasticity limit : WP = 38.27Plasticity index: IP =29.33Ø Sample of Arivonimamo1. Liquid limit WLTable 5. Determination liquid limit of the sample of Arivonimamo
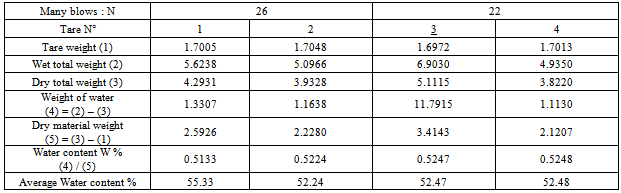 |
| |
|
2 Plasticity limit WP:Table 6. To determine plasticity limit of the sample of Arivonimamo
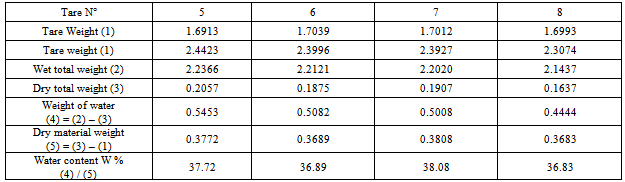 |
| |
|
3. Plasticity index Ip:Liquid limit: Wl = 51.88Plasticity limit : WP =37.38Plasticity index: IP = 14.5Ø Sample for the thermal source of Antsirabe1. Liquid limit W l:Table 7. Determination liquid limit of the sample for the thermal source of Antsirabe
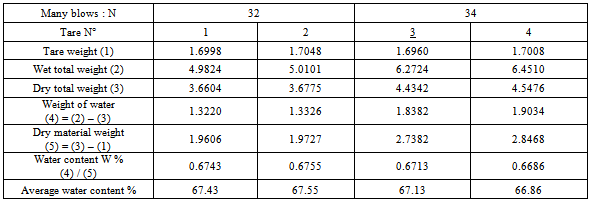 |
| |
|
2. Plasticity limit WP:Table 8. Determination plasticity limit of the sample for the thermal source of Antsirabe
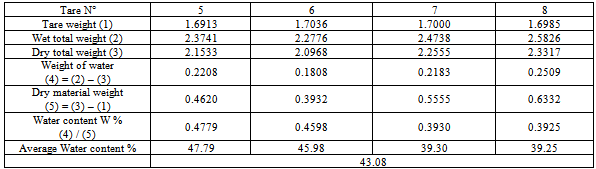 |
| |
|
3. Plasticity index Ip:Liquid limit: W l= 67.24Plasticity limit : WP = 43.08Plasticity index: IP = 24.16.
4.1.2. Chemical Analyses of Clays by X-rays Fluorescence[7,8]
The elements detected by X-ray fluorescence spectrometry of our samples are gathered in the following table: Table 9. Results of the analyses by the method of X-ray fluorescence spectrometry
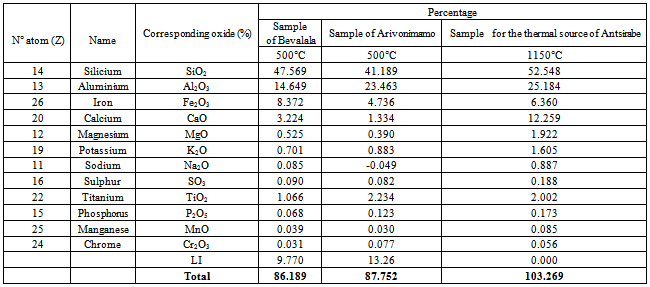 |
| |
|
4.1.3. Improvement of the Content of CaO
We take the clay sample of Arivonimamo for this stage of improvement because this clay has a low plasticity among the three samples. The goal of handling[9, 10] is to increase the percentage of calcium CaO oxide which is an oxide very much used for the clay plasticity.
4.1.3.1. Method
Calcium carbonate is added (CaCO3)[11,12 ,13] of various weight m to clay to be analyzed. We choose the method of X-rays Fluorescence (XRF) to follow the evolution of the contents.
4.1.3.2. Variation of the Content of CaCO3
The results of the analysis by X-ray fluorescence spectrometry are presented in table 10: Table 10. Percentage of the elements detected by the spectrometry of X-ray fluorescence
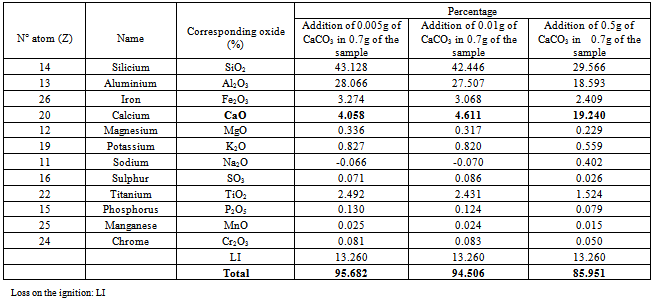 |
| |
|
4.2. Discussion
Some research shows that the consistency of expansive clay soil is strongly influenced by soil water content. According to[14], plasticity index and liquid limit[14] can be used to determine the swelling characteristics of expansive clays in general.[15] stated that the only plasticity index alone[15]can be used to estimate the swelling characteristics of expansive clays in general. According[16], the higher the plastic index[16,17] an expansive clay minerals, the higher the potential for development.The most plastic clays are called “unctuous clay” and those which are the least plastic call “meager clays”[18]. The experiments showed that samples 1 and 3 are much more plastic than sample 2 because the index of plasticity are respectively Ip1= 29.33. Ip2=14.5 and Ip3= 24.16. The analysis by X-ray fluorescence spectrometry of our samples highlights that the major elements are: Na (Z=11), Si(Z= 14) Al(Z=13) Fe(Z= 26) Ca(Z= 20) Mg(Z=12) K(Z= 19) S (Z=16) Ti (Z=22) P (Z= 15) Mn (Z=25) Cr (Z=24). Ne (Z=10) could not be detected by the analysis by X-ray fluorescence spectrometry because Z=10, it is the limit of detection of the detector of the X-ray fluorescence spectrometer.According to the table 10, when we increase the content of CaCO3, the percentage of CaO in the sample increase. The ion Na+ is exchangeable with Ca2+[19]. So we can improve the plasticity of clays by increasing the percentage of CaO.
5. Conclusions
The present study made it possible to get fundamental results on the quantitative and qualitative characteristics of the clay soils in various places of Madagascar. Indeed, the knowledge of plasticity and the chemical components of a clay soil make it possible to determine its use well. Plasticity gave an indication on the quantity of clay in the ground. Sample1 (IP = 29.33) contains much more clay than the two others.By analysis of X-ray fluorescence spectrometry, one can say that the results are in agreement with the percentages quoted in the chemical composition.We take the clay sample of Arivonimamo for the stage of improvement because this clay has a low plasticity among the three samples. The handling consists in adding of CaCO3 to sample 2 (the least plastic) made it possible to conclude that the cations (mono and bivalent) of this sample could be exchanged with of Ca2+ since the content of the CAO in the sample increased and those of the other actions decreased.
References
| [1] | http://fr.wikipedia.org/wiki/ Argile du 19 Mai 2009 |
| [2] | RAZAFIMAHANDRY Armand Andrianirina, «Contribution à l’étude des argiles kaoliniques pour l’élimination des oxydes de fer. Utilisation en industrie céramique et application pédagogique » Mémoire de CAPEN : N 135 PC (105 pages) |
| [3] | RASOAMARINTANY Falilalahaingo (1995) Fabrication des briques et des tuiles artisanales dans la région d’Ambohitrimanjaka, impacts pédagogiques- économiques et sur l’environnement, Mémoire de CAPEN Antananarivo. |
| [4] | Victor BODIN (1956) Technologie des produits de terre cuite Gauthier- Villars Paris (247pages). |
| [5] | P. Sherwood (2004), The healing art of clay therapy, A.C.E.R press, |
| [6] | R. JÜTTE, B. BLESSING (2011), Pathways of homeopathic medicine, springer medizin verlag, Heidelberg |
| [7] | RASOARIMANGA Philippine (2002), Mise en solution du minerai de monazite de Madagascar (Fort Dauphin) caractérisation du minerai et des précipités par : Spectrométrie d’émission UV visible- Spectrométrie de fluorescence « X ». Mémoire de DEA de chimie Antananarivo. |
| [8] | BRINDLEY G.W. (1961), The X-ray identification and cristal structures of clay minerals, Brown, The mineralogical Society, London. |
| [9] | FAUCONNIER P (1967) Cours de chimie générale et de chimie minérale, SEDES, PARIS |
| [10] | CARNOT A (1910) Traité d’analyse des substances minérales, tomes III, DUNOD, PINAT, PARIS |
| [11] | H.SCHOELLER (1934) C.R du congrès d’Erfoud du comité d’Etude des eaux souterraines, p 46-54 |
| [12] | JUNG.J (1933), Ann. Office Nat. Combustibles liquides, n 2, 291-300 |
| [13] | Mc KENZIE Taylor (1928), Journ. of the Institut of Petroleum Technologists, XIV, 835-840 |
| [14] | HOLTZ, R.D., and GIBBS, H.J., Prediction on Swelling Potential for Compacted Clay, Journal of the Soil mechanics and Foundation devisio, ASCE, Discussion, Vol 88, No.SM4, 1956. |
| [15] | SEED, H.B., WOODWARD R.J., LUNDGREN R., (1962) Vol.88. No. SM3. Proc. Paper 3169 “Prediction of Swelling Potential for Compacted Clays”, Journal of the Soil Mechanics and Foundation Division, ASCE, |
| [16] | DAKHSHANAMURTHY, V. and RAMAN, V., “Review of Expansive Soils”, Discusion, Journal of Geotechnical Enggineering Division, ASCE, Volume 101, , No. GT 6, 1975. |
| [17] | MITCHELL, J.K., 1992, Fundamentals of Soil Behavior, Second edition, Jhon Wiley & Sons, Inc., New York, USA |
| [18] | BESAIRIE H. (1960) Monographie géologique de Madagascar in Lexique stratigraphique international Afrique, fascicule Madagascar, Tome 2, Tananarive. |
| [19] | J. R. McHENRY and H. F. RHOADES, (1943), Soil Science Society of American Journal, Vol. 7 No. C, p. 42-47. |

 Here a balance was established but if one systematically renews the solution with balance (by a new solution with balance) by a new solution NaCl, one obtains after several treatments a clay – Na and the number of equivalence of fixed Na+ is equal to that of moved Ca2+. This balance also depends on the concentrations of the exchangeable ions
Here a balance was established but if one systematically renews the solution with balance (by a new solution with balance) by a new solution NaCl, one obtains after several treatments a clay – Na and the number of equivalence of fixed Na+ is equal to that of moved Ca2+. This balance also depends on the concentrations of the exchangeable ions 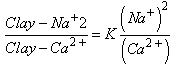 With K= the constant of balance If P is the power of absorption one a:
With K= the constant of balance If P is the power of absorption one a: 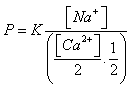 With[Ca2+] and[Na+] are expressed in equivalent grams.During the fixing of the Na+ ions, one can also define solutions used, the capacity of exchange of sodium (sodium adsorption ratio) noted:
With[Ca2+] and[Na+] are expressed in equivalent grams.During the fixing of the Na+ ions, one can also define solutions used, the capacity of exchange of sodium (sodium adsorption ratio) noted: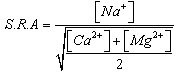 S.A.R is expressed in milliequivalents- gram for clay 100g. Values of the capacities of exchange of the cations and anions are given in the following table:
S.A.R is expressed in milliequivalents- gram for clay 100g. Values of the capacities of exchange of the cations and anions are given in the following table: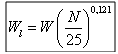 Wl: liquid limit NR: Many blows To calculate Wp (plastic limit). We take a sample of ground and one lets it dry (A). We add a little water and we rolls on a surface punt, a small glass plate for example. We try to form wire a 3 mm thickness and 10 cm length, without it breaking (B). We repeat this experiment by adding each time a little water until you can form a wire. The water content will correspond then to the plastic limit and could be expressed as a percentage weight of the sample.
Wl: liquid limit NR: Many blows To calculate Wp (plastic limit). We take a sample of ground and one lets it dry (A). We add a little water and we rolls on a surface punt, a small glass plate for example. We try to form wire a 3 mm thickness and 10 cm length, without it breaking (B). We repeat this experiment by adding each time a little water until you can form a wire. The water content will correspond then to the plastic limit and could be expressed as a percentage weight of the sample.
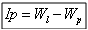 Wl and Wp being liquid limit and plastic limit
Wl and Wp being liquid limit and plastic limit Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML








