-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2013; 3(5): 91-98
doi:10.5923/j.ijmc.20130305.02
In vitro Bioactivity of Polycaprolactone/Bioglass Composites
Lachezar Radev1, Dimitar Vladov1, Irena Michailova2, Ekaterina Cholakova1, Maria F. V. Fernandes3, Isabel M. M. Salvado3
1Department of Fundamental Chemical Technology, University of Chemical Technology and Metallurgy, Sofia, 1756, Bulgaria
2Department of Silicate Technology, University of Chemical Technology and Metallurgy, Sofia, 1756, Bulgaria
3Materials Engineering and Ceramics Department, CICECO, University of Aveiro, 3810-193 AVEIRO, Portugal
Correspondence to: Lachezar Radev, Department of Fundamental Chemical Technology, University of Chemical Technology and Metallurgy, Sofia, 1756, Bulgaria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A series of Polycaprolactone and Bioglass in the 85SiO2-10CaO-5P2O5 (mol. %) systems were synthesized at different quantity of the organic/inorganic components. The 85S Bioglass was prepared via sol-gel method. It was added to the Polycaprolactone matrix at 20, 50 and 80 weight (wt.) %, respectively. In vitro bioactivity of the prepared composites was evaluated in 1.5 Simulated Body Fluid (1.5 SBF). The obtained composite materials before and after static in vitro test were characterized by FTIR and SEM. The obtained experimental data proved that the synthesized composites exhibit good in vitro bioactivity. After immersion in 1.5SBF, SEM depicts the presence of nano-needle-like hydroxyapatite on the immersed 20PCL/80BG and 50PCL/50BG samples.
Keywords: 85SBioglass, Sol-Gel, Polycaprolactone, Composites, In vitro Bioactivity
Cite this paper: Lachezar Radev, Dimitar Vladov, Irena Michailova, Ekaterina Cholakova, Maria F. V. Fernandes, Isabel M. M. Salvado, In vitro Bioactivity of Polycaprolactone/Bioglass Composites, International Journal of Materials and Chemistry, Vol. 3 No. 5, 2013, pp. 91-98. doi: 10.5923/j.ijmc.20130305.02.
Article Outline
1. Introduction
- It is known that Polymer-Glass composites scaffolds enjoy the mechanical reliability of the polymer phase and the excellent bioactivity of the glassy phase[1]. In this context poly (ɛ-caprolactone) (PCL) is used to obtain different bioactive composites, which are already referred in the recent publications, focused on the production and application of PCL-based composites, loaded with different inorganic phases, such as Bioglasses (BG)[2-10], hydroxyapatite[7, 11-16], silica[17, 18], calcium silicate[19], tri calcium phosphate (TCP)[7, 20], ZrO2[21], TiO2[22], forsterite (Mg2SiO4)[23] and hardystonite (Ca2ZnSi2O7)[24]. On the other hand, the promotion role of silicon in bone metabolism was first observed by[25, 26]. Some authors synthesized Si-substituted TCP[27] and hydroxyapatite (Si-HA) with different quantity of silicon[28]. They observed better bioactivity of these ceramics towards bone formation than those without silicon.In the field of PCL/BG composites some authors find that the combinations of PCL/45S BG[2, 3] and PCL/Calcium Silicate BG[2] produce in vitro bioactivity, after soaking of the synthesized composites in SBF for different times. The obtained new precipitate promoted crystals with needle-like morphology. E. Tamij et al.[4] studied when 45SiO2-24.5Na2O-24.5CaO-6P2O5 (wt. %) BG was added to PCL, lower in vitro bioactivity acquired. M. Mohammadi et al.,[5] observed that two types of BG, described as 50P2O5-40CaO-10SiO2 or 10Fe2O3 mixed with PCL promote Brushite formation after soaking in SBF solutions for 14 and 28 days. H-H. Lee and colleagues synthesized in vitro biodegradable composite in the presence of 70SiO2-25CaO-5P2O5 (mol. %) nanofibrous BG and PCL[8]. They concluded that the synthesized composite membranes showed the rapid induction of apatite precipitates on their surface[8]. Furthermore, S. R oohani-Estefani at al., prepared 58SiO2-38CaO-4P2O5 (mol. %) BG/PCL composites[9]. They demonstrated that the shape of the 58BG had significant importance for bone regeneration[9]. B. Lei et al., [10] investigated that the addition of 60SiO2-36CaO-4P2O5 (mol. %) BG enhanced in vitro bioactivity of the investigated composites. Briefly, in spite of numerous publications on PCL/BG composites information on the in vitro bioactivity of PCL/85S BG in 1.5 SBF solutions does not exist. In this study we have synthesized sol-gel glass in the SiO2-CaO-P2O5 system with high silicon content (85 mol. %) and then mixed it with PCL to prepare PCL/BG composites with different content of PCL and BG. PCL was selected, because it has FDA approval in several biomimetic devises, low cost, and most of all, it is slowly degradable. In vitro bioactivity of the prepared PCL/BG composites were evaluated in 1.5 SBF solution.
2. Experimental Part
- Preparation of BG and PCL/BG compositesBG in the SiO2-CaO-P2O5 system was used as filler. BG was synthesized via polystep sol-gel method. The composition was described as 85SiO2-10CaO-5P2O5 (mol. %). The obtained BG was denoted as 85S Bioglass. Tetraethoxysilane (TEOS) and phosphoric acid (H3PO4) were used as sources. CaO was heated at 500℃ for 2 hours before using as Ca(OH)2 in water medium. The synthesis of 85S Bioglass had already been described in[29]. Polycaprolactone (PCL) with Mw=6000 (Sigma-Aldrich) was dispersed in 10 ml chloroform then BG powder was added into PCL solution and continuously stirred for 24 hours to disperse 85S BG uniformly. After that the obtained mixture was heated at 50℃ under vacuum to produce composites. The PCL/BG ratio were fixed as 50:50, 80:20 and 20:80 (wt. %). The synthesized composites were depicted as 50PCL/50BG, 80PCL/20BG and 20PCL/80 BG.Biomimetic surface treatmentIn the presented study, the treatments were conducted in 1.5 SBF solution, to stimulate the nuclei formation on the composite surface, in accordance by the method, described from Kokubo et. al.,[30].The 1.5 SBF was prepared from different reagents as follow: NaCl=11.925 g, NaHCO3=0.5295 g, KCl=0.3360 g, K2HPO4.3H2O=0.3420g, MgCl2.6H2O=0.4575g,CaCl2.2H2O=0.5520g, Na2SO4=0.106 g, and buffering at pH=7.4 at 36.5℃ with 9.0075 g of TRIS and 1M HCl in distilled water. A few drops of NaN3 was added to the 1.5 SBF to inhibit bacterial growth[31]. After soaking, the PCL/BG specimens were removed from the SBF solution, gently rinsed with distilled water and then dried at 36.5℃ for 12 hours.Methods for analysis FTIR spectroscopy was used to identify the functional groups of BG powder and PCL/BG composites with different weight ratio of the components. FTIR spectra were recordered using a Brucker Tensor 27 spectrometer equipped with MCT detector with a resolution of 4 cm-1 (64 scans). KBr pelletized discs containing 2 mg of sample and 200 mg KBr were made. SEM (Jeol, JSM-35CF, Japan) was used to ascertain the morphology and chemical constituents of the prepared composites before and after immersion in 1.5 SBF solution for 12 days at accelerating voltage of 15 kv.
3. Results and Discussion
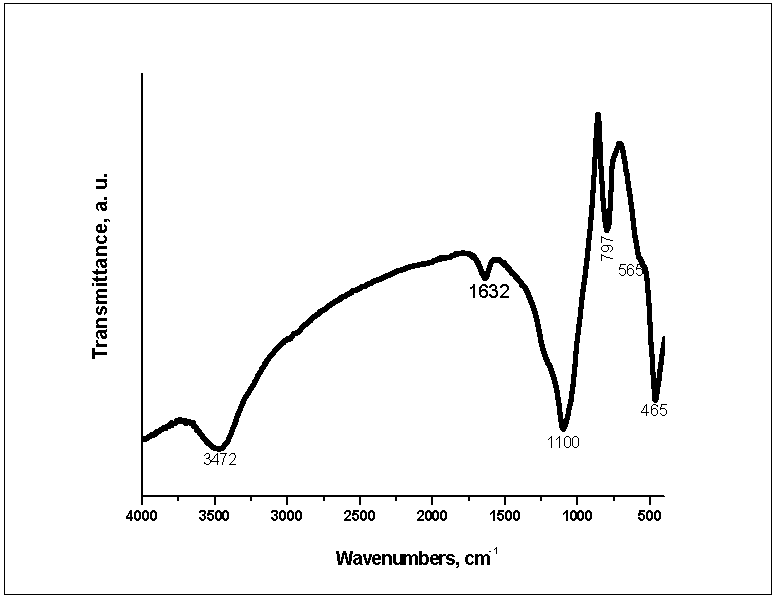 | Figure 1. FTIR of 85S BG |
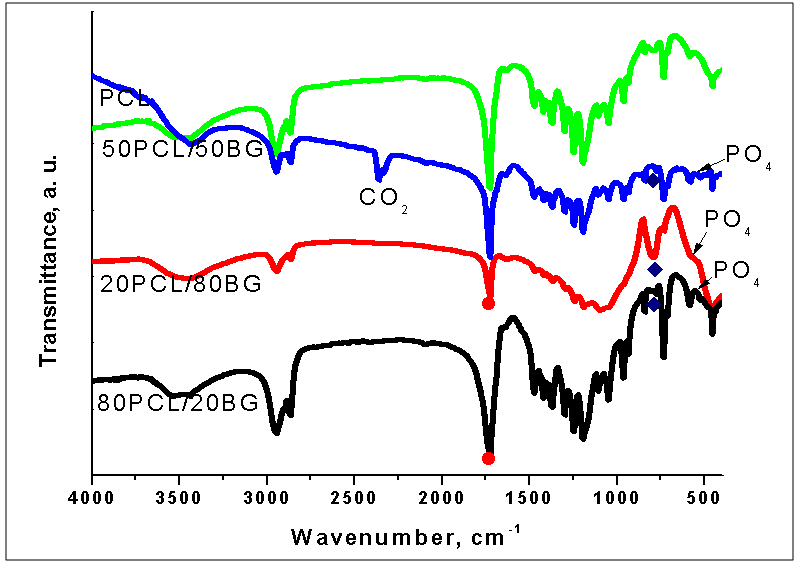 | Figure 2. FTIR of pure PCL and PCL/BG composites at different weight ratio of the components before in vitro test |
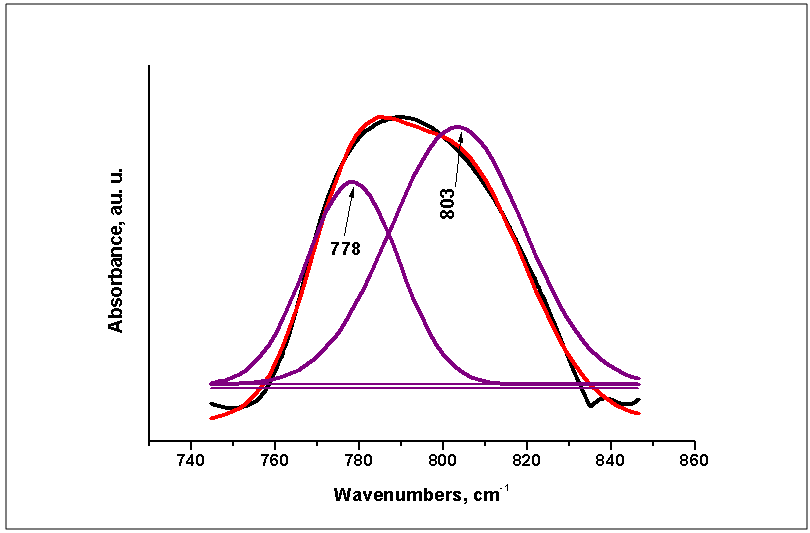 | Figure 3. Curve-fitting spectra of the 50PCL/50BG composite before immersion in 1.5 SBF |
 )[10-12, 23, 34]. Symmetric and asymmetric CH2 stretching mode at 2934 and 2864 cm-1 are also visible[3, 11, 24, 34, 35]. On the other hand, the band at 1294 cm-1 could be ascribed to the backbone C-C and C-O stretching vibrations in the crystalline phase of PCL[23, 24, 34, 35]. The band at ~1167 cm-1 which is assigned to the amorphous phase of PCL[24, 35] is not visible in our case. It is noteworthy the presence of weak absorption peak at 1560 cm-1, attributable to the antisymmetric stretching vibration of the COO- in 50PCL/50BG and 80PCL/20BG composites [13]. Furthermore, the band, centered ~700 cm-1 could be ascribed to the presence of Si-O-Si bond in the sol-gel derived BG[32, 33]. B. Lei et al., proved that in the case of Nanoscale Bioactive Glass (NBG), reinforced PCL composites, the same band also been observed[10]. The presence of this band can be related to the presence of BG into the synthesized composites. As can be seen from the presented in Figure 2 FTIR spectra, the intensity of this band, labeled as
)[10-12, 23, 34]. Symmetric and asymmetric CH2 stretching mode at 2934 and 2864 cm-1 are also visible[3, 11, 24, 34, 35]. On the other hand, the band at 1294 cm-1 could be ascribed to the backbone C-C and C-O stretching vibrations in the crystalline phase of PCL[23, 24, 34, 35]. The band at ~1167 cm-1 which is assigned to the amorphous phase of PCL[24, 35] is not visible in our case. It is noteworthy the presence of weak absorption peak at 1560 cm-1, attributable to the antisymmetric stretching vibration of the COO- in 50PCL/50BG and 80PCL/20BG composites [13]. Furthermore, the band, centered ~700 cm-1 could be ascribed to the presence of Si-O-Si bond in the sol-gel derived BG[32, 33]. B. Lei et al., proved that in the case of Nanoscale Bioactive Glass (NBG), reinforced PCL composites, the same band also been observed[10]. The presence of this band can be related to the presence of BG into the synthesized composites. As can be seen from the presented in Figure 2 FTIR spectra, the intensity of this band, labeled as  , is a function of weight quantity of BG into the composites, i. e. this band is quite visible for the 20PCL/80BG composite (Fig. 2, curve 3).The more detailed information about the presence of PCL and BG in the 50PCL/50BG composite was analyzed by the curve-fitting in the region 745-845 cm-1 (Fig. 3). From the presented Fig. 3 it can be seen that the band, centered at 778 cm-1 could be ascribed to the PCL while the band at 803 cm-1 could be related to Si-O-Si in accordance with FTIR data, presented in Fig. 1.In addition, the well-defined, but not very intense, bands, posited at ~570 cm-1 could be defined as P-O stretching modes of PO43- from 85S BG[36].The surface morphology of the prepared PCL/BG composites before the in vitro test was studied by SEM as shown in Figures 4-6. SEM image of the 50PCL/50BG (Fig. 4) shows that 85SBG has a good dispersion in the PCL matrix. On the other hand, SEM shows the presence of large cavities, due to the removal of chloroform which had previously been introduced during its preparation[11]. The sizes of these cavities are ~40 µm.SEM of the 20PCL/80BG is given in Fig. 5
, is a function of weight quantity of BG into the composites, i. e. this band is quite visible for the 20PCL/80BG composite (Fig. 2, curve 3).The more detailed information about the presence of PCL and BG in the 50PCL/50BG composite was analyzed by the curve-fitting in the region 745-845 cm-1 (Fig. 3). From the presented Fig. 3 it can be seen that the band, centered at 778 cm-1 could be ascribed to the PCL while the band at 803 cm-1 could be related to Si-O-Si in accordance with FTIR data, presented in Fig. 1.In addition, the well-defined, but not very intense, bands, posited at ~570 cm-1 could be defined as P-O stretching modes of PO43- from 85S BG[36].The surface morphology of the prepared PCL/BG composites before the in vitro test was studied by SEM as shown in Figures 4-6. SEM image of the 50PCL/50BG (Fig. 4) shows that 85SBG has a good dispersion in the PCL matrix. On the other hand, SEM shows the presence of large cavities, due to the removal of chloroform which had previously been introduced during its preparation[11]. The sizes of these cavities are ~40 µm.SEM of the 20PCL/80BG is given in Fig. 5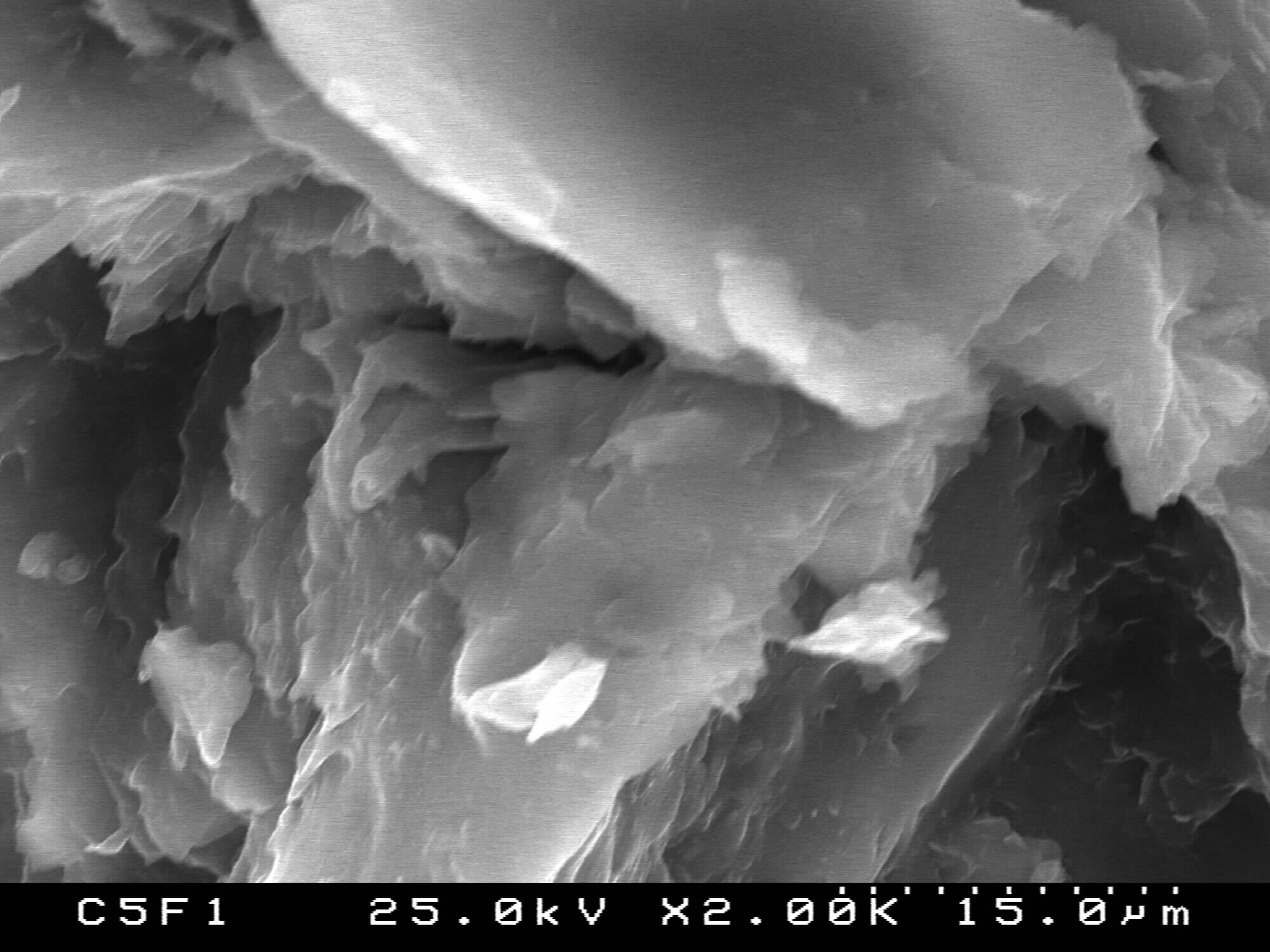 | Fugure 4. SEMfor PCL50/50BG composite |
 | Fugure 5. SEM for 20PCL/80BG |
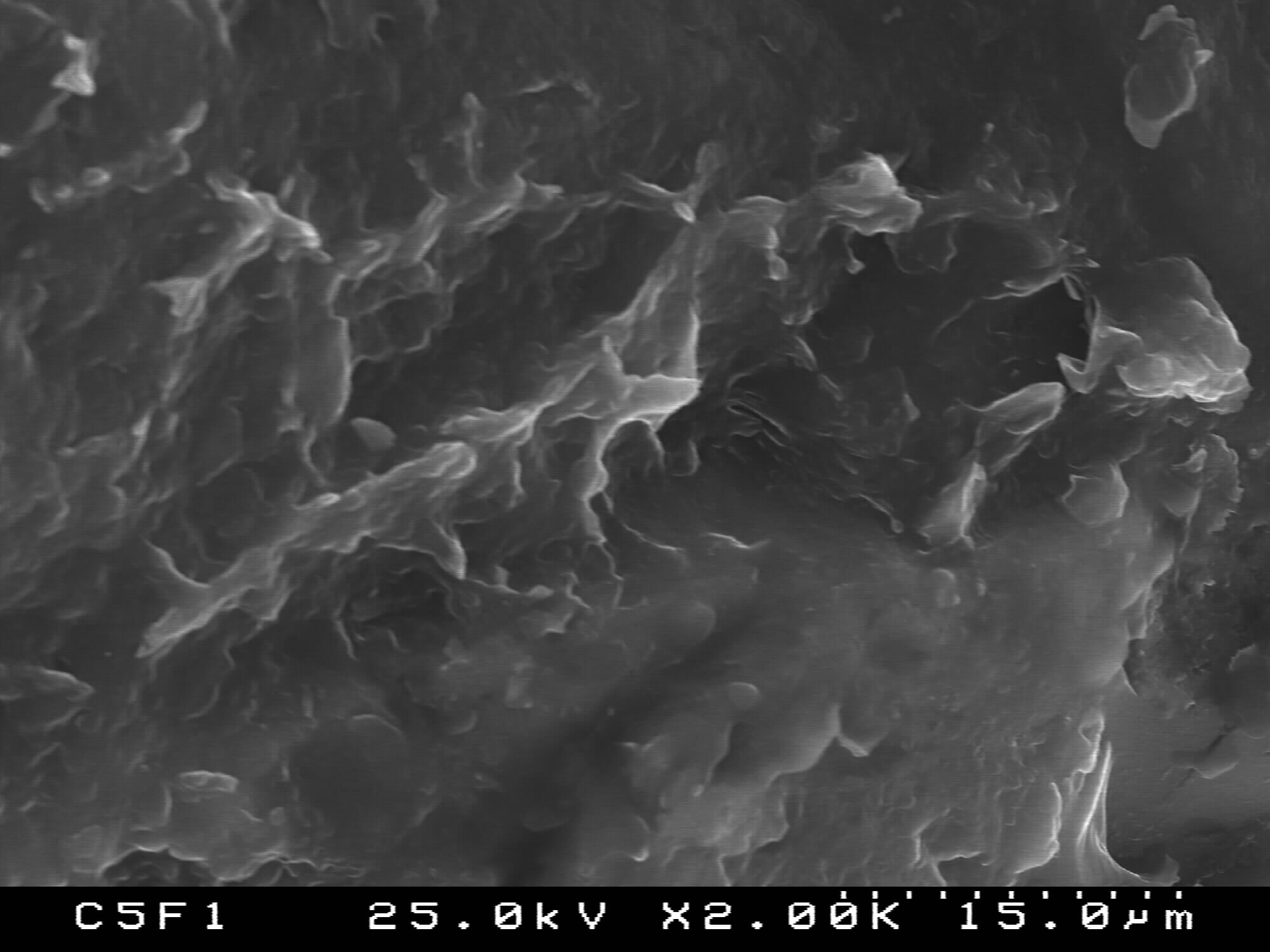 | Fugure 6. SEM of 80PCL/20BG |
 | Figure 7. FTIR of the 50PCL/50BG, 20PCL/80BG and 80PLC/20BG composites, after in vitro test in 1.5 SBF for 3 days in static condition |
 | Figure 8. FTIR of the 50PCL/50BG, 20PCL/80BG and 80PLC/20BG composites after in vitro test in 1.5 SBF for 12 days in static conditions |
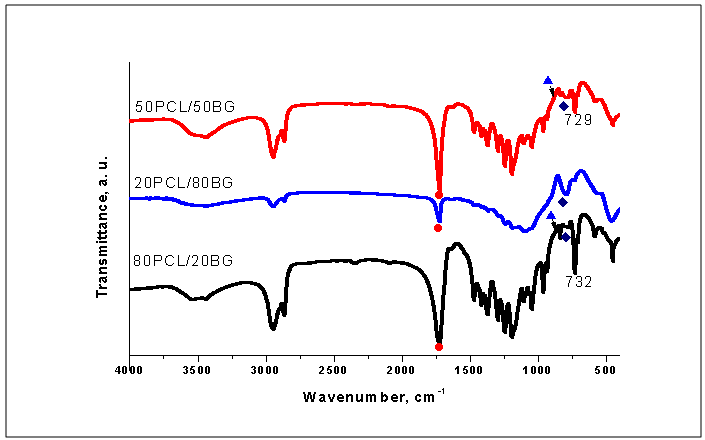
 ). On the other hand, in 80PCL/20BG composite (Fig. 6, curve 3), the intensity of the band at 797 cm-1 (labeled as
). On the other hand, in 80PCL/20BG composite (Fig. 6, curve 3), the intensity of the band at 797 cm-1 (labeled as  ) became more visible. This peak could be ascribed to the Si-O-Si vibration mode of 85S BG (Fig. 1). This fact can be based also on the process of partial dissolution of PCL in 1.5 SBF solution. Furthermore, the P-O stretching modes of PO43- at ~570 cm-1 for the three composites are quite visible. These peaks are indicative for calcium phosphate crystalline phases on the surface of the soaked samples. Moreover, FTIR results presented at Fig. 7 also depict the presence of CO32- bands at 1560, 1462 and 1400 cm-1 (for the 50PCL/50BG), 1530, 1460, 1392 and 1370 cm-1 (for 20PCL/80BG) and at 1436, 1420 and 1400 cm-1 (for 80PCL/20BG). The presence of these bands could be related to the CO3HA formation on the surface of the soaked samples for 3 days in 1.5 SBF solution.From the presented FTIR results we can summarize that:• After in vitro test for 3 days, PCL partially dissolved in to 1.5 SBF solution.• BG does not undergo any visible changes after immersion.• Some CO32- peaks could be observed for the three samples.On the base of these conclusions we can conclude that the synthesized PCL/BG composites are in vitro bioactive. The observed results are in a good agreement with our preliminary studies[29] . FTIR spectra of the prepared PCL/BG composites with different weight ratio of the components after soaking in 1.5 SBF for 12 days are given in Fig. 8.After comparison of the presented at Fig. 7 FTIR spectra with those depicted in Fig. 6 it is quite visible that after 12 days of soaking, the intensity of the characteristic bonds increased. Based on the obtained results, we can conclude that the synthesized composites are covered with a new phase of crystalline calcium phosphates. In this context, the peaks in the 1350-1550 cm-1 region are associated with CO32- moieties (bending ν3 and ν4 modes)[5]. Moreover, the peaks centered at 875 cm-1 (labeled as
) became more visible. This peak could be ascribed to the Si-O-Si vibration mode of 85S BG (Fig. 1). This fact can be based also on the process of partial dissolution of PCL in 1.5 SBF solution. Furthermore, the P-O stretching modes of PO43- at ~570 cm-1 for the three composites are quite visible. These peaks are indicative for calcium phosphate crystalline phases on the surface of the soaked samples. Moreover, FTIR results presented at Fig. 7 also depict the presence of CO32- bands at 1560, 1462 and 1400 cm-1 (for the 50PCL/50BG), 1530, 1460, 1392 and 1370 cm-1 (for 20PCL/80BG) and at 1436, 1420 and 1400 cm-1 (for 80PCL/20BG). The presence of these bands could be related to the CO3HA formation on the surface of the soaked samples for 3 days in 1.5 SBF solution.From the presented FTIR results we can summarize that:• After in vitro test for 3 days, PCL partially dissolved in to 1.5 SBF solution.• BG does not undergo any visible changes after immersion.• Some CO32- peaks could be observed for the three samples.On the base of these conclusions we can conclude that the synthesized PCL/BG composites are in vitro bioactive. The observed results are in a good agreement with our preliminary studies[29] . FTIR spectra of the prepared PCL/BG composites with different weight ratio of the components after soaking in 1.5 SBF for 12 days are given in Fig. 8.After comparison of the presented at Fig. 7 FTIR spectra with those depicted in Fig. 6 it is quite visible that after 12 days of soaking, the intensity of the characteristic bonds increased. Based on the obtained results, we can conclude that the synthesized composites are covered with a new phase of crystalline calcium phosphates. In this context, the peaks in the 1350-1550 cm-1 region are associated with CO32- moieties (bending ν3 and ν4 modes)[5]. Moreover, the peaks centered at 875 cm-1 (labeled as  ) and those at 1415 (1421) cm-1, 1460 (1459) cm-1 could be ascribed to the presence of CO32- into HA lattice, i.e. on the surface CO3HA were formed[14-16]. Furthermore, peaks between 1000-1100 cm-1 are due to the triply generated asymmetric stretching mode of P-O bonds in PO43-[5, 15, 16, 36, 37]. The peak, posited at 960 cm-1 could be ascribed to HPO42-, incorporated into the lattice. This peak could be ascribed to the dicalcium phosphate dihydrate (DCPD or Brushite) on the soaked surface after 12 days of immersion. Based on the presented FTIR results we can conclude that on the surface of the prepared PCL/BG composites, two types of products can be formed –DCPD and CO3HA.SEM of the immersed samples in 1.5 SBF solution after 12 days of soaking are given in Fig.9
) and those at 1415 (1421) cm-1, 1460 (1459) cm-1 could be ascribed to the presence of CO32- into HA lattice, i.e. on the surface CO3HA were formed[14-16]. Furthermore, peaks between 1000-1100 cm-1 are due to the triply generated asymmetric stretching mode of P-O bonds in PO43-[5, 15, 16, 36, 37]. The peak, posited at 960 cm-1 could be ascribed to HPO42-, incorporated into the lattice. This peak could be ascribed to the dicalcium phosphate dihydrate (DCPD or Brushite) on the soaked surface after 12 days of immersion. Based on the presented FTIR results we can conclude that on the surface of the prepared PCL/BG composites, two types of products can be formed –DCPD and CO3HA.SEM of the immersed samples in 1.5 SBF solution after 12 days of soaking are given in Fig.9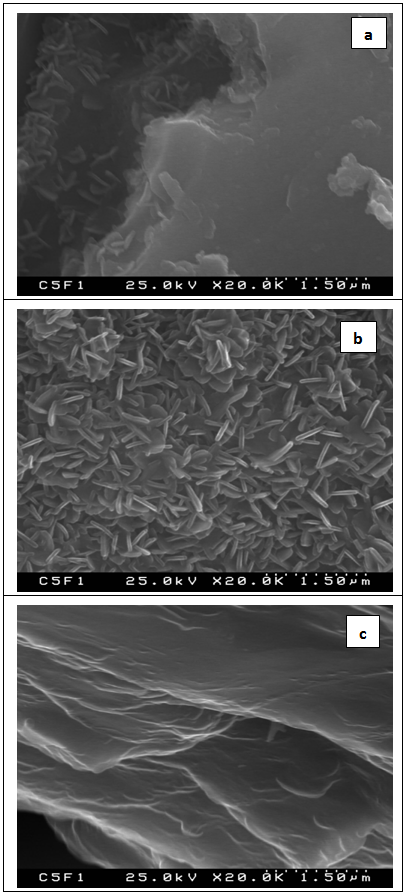 | Figure 9. SEM of the 50PCL/50BG (a) 20PCL/80BG (b) and 80PLC/20BG (c) composites after in vitro test in 1.5 SBF for 12 days in static conditions |
4. Conclusions
- The PCL/BG composites have been prepared. BG was synthesized via sol-gel method using TEOS, CaO and H3PO4 as sources. The nominal composition of BG was 85SiO2-10CaO-5P2O5 (mol. %). PCL/BG composites were synthesized by mixing of PCL and BG at 20PCL/80BG, 50PCL/50BG and 80PCL/20BG weight ratio. In vitro bioactivities of the synthesized composites were examined in 1.5 SBF solutions for 3 and 12 days in static conditions. The obtained samples were characterized by FTIR and SEM. FTIR of the samples before in vitro test showed that BG was included in to PCL matrix. After 3 days of soaking in 1.5 SBF solution FTIR observed that the intensity of the characteristic bands of PCL significantly decreased. This result can be attributed to the process of partial dissolution of the organic part of the composites. On the other hand, some new carbonate bands could be observed. The presence of these new groups could be related to the carbonate containing hydroxyapatite, crystallized on the immersed sample. After 12 days of soaking, FTIR revealed the formation of DCPD and HA on the surfaces of the immersed samples. The obtained results depict that the synthesized PCL/BG composites are in vitro bioactive. SEM of these samples presented that needle-like HA can be observed. Based on the presented results we can conclude that the prepared PCL/BG samples exhibit good in vitro bioactivity.
ACKNOWLEDGEMENTS
- The authors would like to acknowledge the Programme PEst-C/CTM/LA0011/2013 for finding this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML