E. A. Kamba, A. U. Itodo, E. Ogah
Department of Chemical Sciences, Federal University Wukari Nigeria
Correspondence to: A. U. Itodo, Department of Chemical Sciences, Federal University Wukari Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The ratio of emulsifying agents used to achieve stability is very important. In this study effect of surfactant HLB and concentration on the emulsion stability were investigated. The time required for the two liquids to separate, creaming volume and microscopic observation were used to assess the emulsion stability. Emulsifiers used in this study are based on their chemical structures and include synthetic, natural and finely dispersed solid particles emulsifiers. It was observed that the optimal surfactant concentration for oil/water emulsion long-term stability were 20% wt/vol. soap in the oil phase and 0.1% wt/vol. detergent in the continuous phase. Higher concentration of soap had a destructive effect on oil/water emulsion stability which correlated with the observation that interfacial film strength at the oil/water interface decreases as the detergent concentration increases. Methanol added to the inner aqueous phase exerted an osmotic pressure that caused diffusion of oil into aqueous phase and increased oil/water emulsion viscosity through an increase in the volume fraction of the primary oil/water emulsion. These types of viscosity increase impose a destabilization effect because of the likelihood of rupture of the outer and continuous phase droplets.
Keywords:
Emulsifiers, Surfactant, Soap, Detergent, Emulsifying Agents, Stabilization, Emulsions
Cite this paper: E. A. Kamba, A. U. Itodo, E. Ogah, Utilization of Different Emulsifying Agents in the Preparation and Stabilization of Emulsions, International Journal of Materials and Chemistry, Vol. 3 No. 4, 2013, pp. 69-74. doi: 10.5923/j.ijmc.20130304.01.
1. Introduction
Emulsions are used as a basis for a wide variety of both naturally occurring as well as manufactured materials in the industries such as food industries, pharmaceutical industries and cosmetic industries. Others include agrochemicals, petrochemicals and explosives[1].Stability in emulsions is very important as it forms the basic approach for providing solution to problems in the manufacture of foods, drugs and cosmetics. The rheological, physicochemical and nutritional properties of some systems such as foods can be improved by regularly incorporating ingredients used in the process of emulsion preparation[2]. Stabilization of emulsion becomes necessary in order to avoid loss of activity, degradation, and even reaction with some components present in food systems of theseingredients which can lead to limitation in their bio-availability, or change theircolor or taste[2]. In general, emulsions are by nature physically unstable, that is, they tend to separate into two distinct phases or layers over time. Any emulsion in which the globules do not retain their initial character and do not remain uniformly distributed throughout the continuous phase is said to beunstable.Such emulsion would exhibit different character other than the ideal behavior[2]. The types of instability in emulsions include; Creaming, Breaking or CrackingandCoagulation or Flocculation. Although some pairs of liquids are immiscible, they can be forced together in an emulsion. Instead of forming two separate layers with a clear boundary between them, small droplets of one liquid are spread throughout the other liquid. This is achieved by using an emulsifying agent[3].
1.1. Hydrophile-lipophile-balance (HLB) System
A system was developed to assist in making systemic decisions about the amount and types of surfactants needed in stable products. The system is called the HLB system and has an arbitrary scale of 1-18. HLB numbers areexperimentally determined for the different emulsifiers. If an emulsifier has a low HLB number, there are a low number of hydrophilic groups on the molecule and it will have more of a lipophilic character. For instance, substances with low HLB numbers are generally oil soluble. As a result of their oil soluble character, they will cause the oil phase to predominate and form a water-in-oil emulsion. The higher HLB numbers would indicate that the emulsion has a large number of hydrophilic groups on the molecule and therefore should be more hydrophilic in character. Substances with high HLB numbers are water-soluble. And because of their water soluble character, they will cause the water phase to predominate and form an oil-in-water emulsion.Combination of emulsifiers can produce more stable emulsions than using a single emulsifier with the same HLB number[4].Although many works have been carried out aimed at testing the time required for two liquids to separate after being forced together by means of various emulsifiers, different ways were followed to achieve this. Jim and Diane investigated the stability of water-in-water multipleemulsions by treating with span 83 and Tween 80. Rheological measurements were carried out using an AR 1000 Rheometer. Rotational mapping was performed to eliminate possible small variations caused by the uneven surface of the shaft. A continuous ramping flow mode was used to measure viscosity under controlled shear stress ranging from 0.01 to 100 Pa. An Oscillation procedure at constant frequency of 1Hz was used to obtain information on storage and loss moduli. The information regarding the multiple droplet sizes was obtained by taking photomicrographs of the emulsion samples. The first step that is, the preparation of primary emulsion was carried out in a high shear device to produce very fine droplets. The second emulsification step was carried out in a low-shear device to avoid rupturing the multiple droplets[5]. Other methods of determining the emulsion stability have been developed by researchers. These include; droplet size analyses[4], measuring physical properties of emulsion[5], accelerated tests[6], and light scattering[7].The objective of this study was to evaluate the long-term stability and creaming volume of oil/water emulsion with respect to the concentration, and HLBs of surfactants used.The objective of this study was to evaluate the long-term stability and creaming volume of oil/water emulsion by measuring the concentration of emulsifiers, HLBs of surfactants used, and Turbidity. Evaluation of the effect of some formulation variables like, the emulsifier type and oil phase content on emulsion stability was also carried out.
2. Experimental
2.1. Materials
Lecithin, phospholipids derived from egg yolk and consisting primarily of phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol, a product of M & B England, wasobtained as a solution. Cholesterol was also a product of M & B England. Analytical grade magnesium hydroxide,[Mg(OH)2] and aluminium hydroxide,[Al(OH)3] were products of BDH Chemicals Ltd. England. Methanol was manufactured by Merk Germany. Olive oil, soap and detergent which are products of MZM Continental Company Nigeria and Unilever Nigeria Plc respectively, were obtained from local stores. Distilled water was used throughout while Starch, a product of Ficko Manufacturing Company, FRN, was in powder form. Others reagents used include acetic acid and NaN3.Routine laboratory apparatus were utilized viz: beakers, microscope(Nikon microscope Eclipse E400, Nikon Corporation, Japan), test tubes, micro-slides, grease pencil, cover-slips, stop watch, electronic balance (Accu-622, Fisher Scientific, Fair Lawn, New Jersey, USA), blender, measuring cylinder and mortar and piston.
2.2. Preparation of Soap Solution
20g of soap was grounded and dissolved in 50cm3 of distilled water. 8 test-tubes were set. Each was marked with a grease pencil 3mm above the bottom curvature of the test-tubes. 50cm3 of olive oil was measured in a beaker[6].
2.3. Preparation of Lecithin Solution Emulsifier
Buffer solution of acetic acid and NaN3was prepared by dispersing in a proportion of 100 mM and 0.02 wt% respectively in water and the pH was then adjusted to 3.0 by adding HCl. To prepare lecithin emulsifier, 2.0 wt% lecithinwas dispersed into the buffer solution and was blended for 1 min at a frequency of 20 kHz, amplitude of 70%, and duty cycle of 0.5second to ensure complete dispersion of the emulsifier. The pH of the solution was maintained at 3.0 by addingHCl and then the solution was stirred for about 1 hour.
2.4. Preparation of Emulsion
Two sets of oil-in-water emulsion were prepared; Emulsion 1: 20g of starch powder was levitated in a mortar, with 20cm3 oil until the powder was thoroughly wetted, then 10cm3of distilled water was added all at once, and the mixture was vigorously and continually titrated for 3minutes until the primary emulsion formed was creamy white and produced a “cracking” sound. Additional 20cm3 water was incorporated after the primary emulsion was formed. 10 drops of soap solution was incorporated directly into the primary emulsion, and addition of 10cm3 of methanol was next. When all the agents were incorporated, the emulsion was brought to final volume in a measuring cylinder and then blended to ensure uniform distribution of ingredients[5].The second form ofemulsion (emulsion 2) was prepared by homogenizing 20 wt% olive oil and 1.6 wt% of the aqueous lecithin solution for several minutes using a stirring bar followed by blending. The emulsion was finally collected into a beaker and weighted on an electronic balance (Accu-622, Fisher Scientific, Fair Lawn, NJ, USA), connected to a personal computer to record time and mass data every 2 seconds using an installed data acquisition software (AccuSeries USB version 1.2, Fisher Scientific, Fair Lawn, NJ, USA); The experiments were carried out at 19.7℃.
2.5. Testing Emulsifying Strengths
2.5.1. Using Synthetic Emulsifiers
3 test tubes were set, to one, 10cm3 of distilled water waspoured, 5cm3 of olive oil was added. 10 drops of soap solution were added. To the second test–tube 10cm3 of distilled water and 5cm3 of olive oil were shaken and 10 drops of detergent were added. To the third 10cm3 of distilled water was poured followed by 5cm3 of olive oil and mixture of soap solution and detergent were added in drops (10). The final volume of liquid in each test-tube was approximately the same. The test-tubes were shaken for 2 minutes and then left to stand. Time of separation of the liquids in each test-tube was noted[6].
2.5.2. Using Natural Emulsifiers
3 test-tubes were set; to each 10cm3 of distilled water and 5cm3of olive oil were added. To the first 10 drops of cholesterol were added, to the second 10 drops of lecithin were added and to the third 10 drops of the mixture of cholesterol and lecithin were added. The 3 test-tubes were shaken and left to stand. Time of separation of the liquids in each test-tube was recorded[6].
2.5.3. Using Finely Dispersed Solid Particle Emulsifiers
In 2 test-tubes, 10cm3 ofdistilled water and 5cm3 of olive oil were poured. To the first, drops of magnesium hydroxide were added and to the second 10 drops of aluminum hydroxide were added. The test-tubes were shaken and time of separation in each was recorded[6].
2.6. Effects of Surfactant HLB on Emulsion Stability
8 test-tubes were set to each 5cm3 of olive oil and 5cm3 of distilled water were added, drops of soap solution were added to the tubes as indicated in table 3. Drops of detergent were also added to the tubes as indicated in the same table. Each test-tube was shaken vigorously for 30-45 seconds. The time required for the interface to rise to the grease pencil mark was recorded. The tubes were ranked from 1-8 that is, from the longest to the shortest time of separation. The HLB value for the surfactant system in each test-tube was calculated using the relation below[7].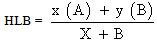 | (1) |
x is Quantity of surfactant 1, A is HLB of Surfactant 1, y is Quantity of surfactant 2 and B is HLB of Surfactant 2.
2.7. Effect of Surfactant Concentration on Emulsion Stability
The steps in 2.5 above were repeated but using 3 times the amounts of soap solution and detergent as indicated in table 4[7].
2.8. Creaming Volume Measurement
The volumes of the creamed phase and the remaining part of the emulsion were recorded. The following relation was used to calculatethe creaming volume: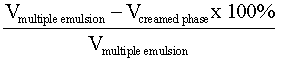 | (2) |
This was achieved by pouring in a measuring cylinder[8].
2.9. Microscopic Observation
The effect of lecithin used in both emulsions was analyzed at different times by gently agitating each emulsion in a test tube before analysis. A drop of each emulsion was placed on a microscope slide and thencovered with a cover slip. The microstructure of the emulsion was then observed usingconventional optical microscopy (Nikon microscope Eclipse E400, Nikon Corporation, Japan). The images were obtained using a CCD camera (CCD-300T-RC,DAGE-MTI,Michigan City, IN) with Digital Image Processing Software (Micro VideoInstruments Inc., Avon, MA) installed on a computer.
3. Results and Discussion
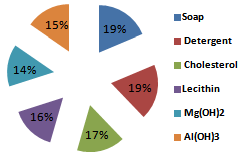 | Figure 1. Percent creaming volume foroil/water emulsion containing various emulsifiers |
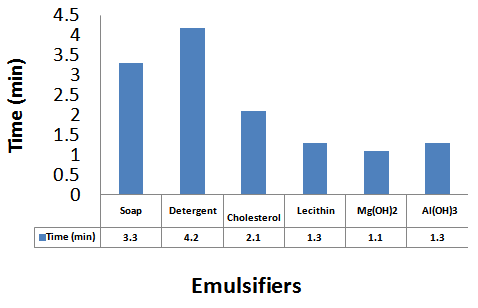 | Figure 2. Separation time (min) for oil/water emulsion containing various emulsifiers |
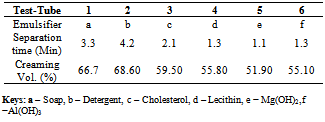
Table 1. Separation time and creaming volume for oil/water emulsion containing single emulsifiers
 |
| |
|
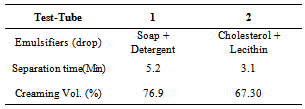
Table 2. Separation time and creaming volume for oil/water emulsion containing mixed emulsifiers
 |
| |
|
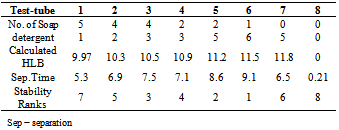
Table 3. Effect of surfactant HLB on emulsion stability
 |
| |
|
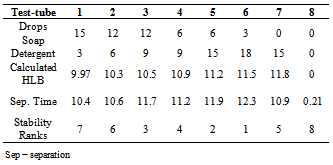
Table 4. Effect of surfactant concentration on emulsion stability
 |
| |
|
3.1. Discussion
The formulation of emulsion 1 was well blended to ensure uniform distribution of ingredients. The methanol might reduce the physical stability of the emulsion, so it is added as near to the end of the process as possible to avoid breaking the emulsion by precipitating the starch powder. After 2 weeks, the emulsion was observed to form 2 layers, one containing white substance which settled at the bottom of the container and the other containing a partially clear solution. This indicates that if the emulsion is left to stand for a long period of time, the partially clear solution may be clearer, that is more ingredients must have settled down. The soap solution that was added is believed to have broken down the oil molecules into smaller ones.Figure 1 is typical of the Percent creaming volume foroil/water emulsion containing various emulsifiers. Volume recorded for soap and detergent is higher than those presented for other emulsifying agents. This is an indication that they are better emulsifiers.Figure 2 represents Separation time (min) for oil/water emulsion containing various emulsifiers. Here, the role of emulsifying agents on separation time was observed. Detergents emulsion present high separation time of 3.3 minutes. This implied that detergent emulsion is more stable. The trend of stability followed the order; detergent (4.2 minutes) > Soap (3.3min) > Cholesterol (2.1 min) >Lecithin (1.3 min)>Al(OH)3(1.3 min)>Mg(OH)2 (1.1 minutes).Table1 contains the proportions of water and olive oil in which soap and detergent were used as emulsifiers. Soap contains a sodium carboxylate salt (R-COO- Na+) which is highly ionic and usually quite water-soluble because of the strong attraction of water molecules to the charges on the ions. The R-group is a long hydrocarbon chain, as in the sodium stearate used. The structure is as follows[8].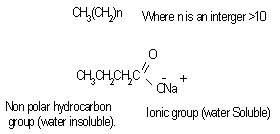 These diagrams symbolized the two important features of the structure. The circle represents the ionic carboxylates end of the molecule and the long time represents the non-polar hydrocarbon chain. The non-polar hydrocarbon group is not soluble in water, but water would be attracted to the ionic groups and hence tend to dissolve the molecules. As soon as the soap molecules are added, the hydrocarbon portions will not permit them to be exposed to water. Instead, they are attracted to each other, forming a cluster in which they are literally dissolvedin each other. This grouping allows the ionic groups at the ends of the soap molecules to be attracted to the surrounding water molecules. The result is that the soap molecules are, in a sense, able to dissolve in the water.The agitation helps jar the oil from the surface and disperse it into tiny droplets. More and more soap molecules surround the oil, until it becomes incorporated within a soap micelle. An emulsion is achieved at this level.In table 1, it took soap 3.3mins to separate water and oil, while detergent took 3.2mins and their mixture 6.5mins this indicates that detergents are better emulsifiers than soap and their mixture more better emulsifiers. Cholesterol proved better emulsifying agents than lecithin and their mixture more stronger emulsifiers. Mg(OH)2 is a less powerful emulsifying agent than Al(OH)3. Detergent on the other hand has molecules with features they share with soap. They are amphipathic and have a large non- polar hydrocarbon end that is oil soluble and a polar end that is H2O soluble. They act in essentially the same way as soap does[9]. Mixture of soap and detergent gave a more stable emulsion, increasing the charges on the oil droplets this keeps them from coalescing[8].Cholesterol and lecithin have effect on interfacial tension, but exert a proactive colloid effect reducing the potential for coalescence by providing a protective sheath around the droplets, impacting a charge to the dispersed droplets. Cholesterols give oil the capacity to absorb water; this is attributed to the little time of separation between the liquids. Lecithin on the other hand has a strong hydrophilic character[10]. When Cholesterol and lecithin were mixed, lycolecithin and cholersetyl ester were formed which are the real substances doing dissolution of oil in water[9]. When Mg(OH)2 was added it only took 1.1min for the layer between the molecules to appear again, and when Al(OH)3 was added it took 1.8min to separate. This is an indication that the two emulsifiers are poor in dissolution character. However Al(OH)3 is a better emulsifier than Mg(OH)2.Flocculation and resultant creaming represent potential steps towards complete coalescence of the dispersed phase. In addition the creaming volume is indicative of the stability of internal aqueous droplets entrapped in the emulsifier droplet, since swelling or shrinkage of the internal aqueous drops directly affect the oil droplets size and hence thee creaming volume[12].Table 3 contains the result obtained when effect of surfactant HLB was tested. It can be seen from the table that the HLB values increased from test-tube 1-7. Test-tube 8 had a zero HLB because no surfactant was added. The time of separation elapsed before the first signs of phase separation was observed, and the stability was ranked from 1, (longest time) to 8, (shortest time). At low soap and detergent concentration, rapid coalescence among the inner and outer droplets to inner phase occurred, resulting in separation within a short period of time. In table 3, Test-tube number 6 took the longest time to separate the liquids thus the highest stabilized. As time of separation decreases the stability increases.
These diagrams symbolized the two important features of the structure. The circle represents the ionic carboxylates end of the molecule and the long time represents the non-polar hydrocarbon chain. The non-polar hydrocarbon group is not soluble in water, but water would be attracted to the ionic groups and hence tend to dissolve the molecules. As soon as the soap molecules are added, the hydrocarbon portions will not permit them to be exposed to water. Instead, they are attracted to each other, forming a cluster in which they are literally dissolvedin each other. This grouping allows the ionic groups at the ends of the soap molecules to be attracted to the surrounding water molecules. The result is that the soap molecules are, in a sense, able to dissolve in the water.The agitation helps jar the oil from the surface and disperse it into tiny droplets. More and more soap molecules surround the oil, until it becomes incorporated within a soap micelle. An emulsion is achieved at this level.In table 1, it took soap 3.3mins to separate water and oil, while detergent took 3.2mins and their mixture 6.5mins this indicates that detergents are better emulsifiers than soap and their mixture more better emulsifiers. Cholesterol proved better emulsifying agents than lecithin and their mixture more stronger emulsifiers. Mg(OH)2 is a less powerful emulsifying agent than Al(OH)3. Detergent on the other hand has molecules with features they share with soap. They are amphipathic and have a large non- polar hydrocarbon end that is oil soluble and a polar end that is H2O soluble. They act in essentially the same way as soap does[9]. Mixture of soap and detergent gave a more stable emulsion, increasing the charges on the oil droplets this keeps them from coalescing[8].Cholesterol and lecithin have effect on interfacial tension, but exert a proactive colloid effect reducing the potential for coalescence by providing a protective sheath around the droplets, impacting a charge to the dispersed droplets. Cholesterols give oil the capacity to absorb water; this is attributed to the little time of separation between the liquids. Lecithin on the other hand has a strong hydrophilic character[10]. When Cholesterol and lecithin were mixed, lycolecithin and cholersetyl ester were formed which are the real substances doing dissolution of oil in water[9]. When Mg(OH)2 was added it only took 1.1min for the layer between the molecules to appear again, and when Al(OH)3 was added it took 1.8min to separate. This is an indication that the two emulsifiers are poor in dissolution character. However Al(OH)3 is a better emulsifier than Mg(OH)2.Flocculation and resultant creaming represent potential steps towards complete coalescence of the dispersed phase. In addition the creaming volume is indicative of the stability of internal aqueous droplets entrapped in the emulsifier droplet, since swelling or shrinkage of the internal aqueous drops directly affect the oil droplets size and hence thee creaming volume[12].Table 3 contains the result obtained when effect of surfactant HLB was tested. It can be seen from the table that the HLB values increased from test-tube 1-7. Test-tube 8 had a zero HLB because no surfactant was added. The time of separation elapsed before the first signs of phase separation was observed, and the stability was ranked from 1, (longest time) to 8, (shortest time). At low soap and detergent concentration, rapid coalescence among the inner and outer droplets to inner phase occurred, resulting in separation within a short period of time. In table 3, Test-tube number 6 took the longest time to separate the liquids thus the highest stabilized. As time of separation decreases the stability increases. | Figure 3. globules from Lecithin+ Cholesterol Emulsion |
Table 4, represents the effect of surfactant concentration on emulsion stability. Effect of increasing the concentration of the surfactants was measured. It can be observed that the HLB values remained the same while the time of separation increased. Hence, the ranking changed. Stability of emulsion can be tested for using other means. All these depend on the availability of materials[10]. As HLB value are scaled 1-8, soaps and detergents have 9.6 and 11.8 respectively. Their HLB values give them the ability or capacity to be both oil and water soluble. But their combination produced more stable emulsion than using them singly; with the same HLB values[10]. However, HLB of 11.5 produces the most stable emulsion of water and oil. On increasing the concentration of surfactants in table 4, the time of separation also increased, therefore, the more concentration of surfactant the more stable an emulsion is, also the longer the time it takes for separation of phase the more stable the emulsion is.When emulsions are stable they tend to have smaller globules than unstable or less stable emulsions[17]. Figures 3-6 show globules formed when different types of emulsifiers were used. A combination of lecithin and cholesterol gave bigger globules than lecithin only (Figure 3). Similarly,combination of soap and detergent formed smaller globules than soap only (Figures 4 & 5) In general, findings from this study is in good agreement with those reported in existingliteratures[13,14,15,16].  | Figure 4. globules from Soap Emulsion |
 | Figure 5. globules from Soap +Detergent Emulsion |
 | Figure 6. globules from Lecithin emulsifier |
4. Conclusions
Emulsions are very important mixtures, their stability study are still more important. In this study, the stability of emulsions depends on the type and proportion of emulsifiers used. Findings from this analysis revealed that on increasing the concentration of surfactants, the time of separation also increased, therefore, the more concentration of surfactant the more stable the emulsion is. Also, the longer the time it takes for separation of phase the more stable the emulsion is. Measure of synergistic effect proved that mixture of soap and detergent gave a more stable emulsion. The microscopic pictures of both emulsions indicate that lecithin used in emulsion 2 (Figure 6) has greater stabilizing power than that used in emulsion 1 (Figure 3). This can be seen in the sizes of emulsion globules which tend to be bigger in emulsion 1 (Figure 3).
References
| [1] | M. Shahin, S.A. Hady, M. Hammad and N.Mortadadevelopment of stable O/W emulsions of three different oils. International Journal of Pharmaceutical Studies and Research Vol. II/ Issue II, 45-51, 2011 |
| [2] | J. Surha, Y.G. Jeongb and G.T. Vladisavljević On the preparation of lecithin-stabilized oil-in-water emulsions by multi-stage premix membrane emulsification |
| [3] | N.Akhtar1, M.Ahmad, H.M.S. Khan, J.Akram, Gulfishan,A. Mahmood and M.Uzairformulation and characterization of a multiple emulsioncontaining 1% l-ascorbic acid.Bull. Chem. Soc. Ethiop. 2010, 24(1), 1-10. |
| [4] | F.Donsì, M. Annunziata, G. Ferrari Microbial inactivation by high pressure homogenization: Effect of the disruption valve geometry Journal of Food Engineering 115 (2013) 362–370 |
| [5] | I.A. Mersah, G.G Michael, T. fabian, M. Osuogi. A Level Chemistry, West African Publishers. Afran Publications, Ghana Ltd, Pg. 186-196, 1993. |
| [6] | K.K Sharma, L.K Sharma. A Text Book of Physical Chemistry, Vikab Publishing House PVT Ltd. Pg. 545-546, 2002 |
| [7] | A.L Williams, H.D Embree, H.J De Bey. Introduction to Chemistry, Addison-Wesley Publishing Company London. Pg. 170-173, 1968 |
| [8] | J.GNairm. Solutions, Emulsions, Suspensions and Extracts, London University Press.Pg. 1509-1515, 2000. |
| [9] | J. Swarbrick. Course Dispersions.Bacon Inc. Boston. Pg. 282-290, 2002 |
| [10] | W.J Reilly. Pharmaceutical Necessities, Oxford University Press, Richard Clay Ltd. Bungay Suffolk. Pg. 1395-1399, 1992. |
| [11] | W. How, K.D. Papadopoulos.Physiocochemical and Engineering Aspects. Washington pg. 181-187, 1997. |
| [12] | J. Jiao and D.J Burgess.Rheology and Stability of Water-in-oil-in-water Multiple Emulsions(http://www.pharmsci.org) , 2003. |
| [13] | M. Mooney ,The Viscosity of a Concentrated Suspension of Spherical Particles. London University Press. Pg. 162-170, 1951 |
| [14] | G.LZubay, W. W Parson, D. E Vance. Principles of Biochemistry Wm. C. Brown Publishers Inc. united States. Pg. 465-476, 1995 |
| [15] | C. Donald, G. Allyn. Non Aqueous Solvents.Macmillan Publishers, New York. Pg. 122-125, 1992 |
| [16] | P. Walstra, Dekker. Encyclopaedia of Emulsion Technology. Vol. 4.Pg 1-56, 1996. |
| [17] | S.B. Daniela, A.P.Tatiana, R.M Naira,B. Josiane,., S.V.Gisely, C.O.Gustavo and A. R. Pedro, Formation and stability of oil-in-water nanoemulsions containing rice bran oil: in vitro and in vivo assessments . Journal of Nanobiotechnology, 9:44, 2011 |

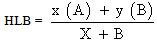
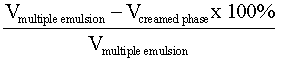


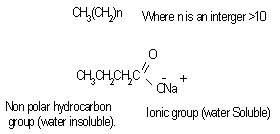 These diagrams symbolized the two important features of the structure. The circle represents the ionic carboxylates end of the molecule and the long time represents the non-polar hydrocarbon chain. The non-polar hydrocarbon group is not soluble in water, but water would be attracted to the ionic groups and hence tend to dissolve the molecules. As soon as the soap molecules are added, the hydrocarbon portions will not permit them to be exposed to water. Instead, they are attracted to each other, forming a cluster in which they are literally dissolvedin each other. This grouping allows the ionic groups at the ends of the soap molecules to be attracted to the surrounding water molecules. The result is that the soap molecules are, in a sense, able to dissolve in the water.The agitation helps jar the oil from the surface and disperse it into tiny droplets. More and more soap molecules surround the oil, until it becomes incorporated within a soap micelle. An emulsion is achieved at this level.In table 1, it took soap 3.3mins to separate water and oil, while detergent took 3.2mins and their mixture 6.5mins this indicates that detergents are better emulsifiers than soap and their mixture more better emulsifiers. Cholesterol proved better emulsifying agents than lecithin and their mixture more stronger emulsifiers. Mg(OH)2 is a less powerful emulsifying agent than Al(OH)3. Detergent on the other hand has molecules with features they share with soap. They are amphipathic and have a large non- polar hydrocarbon end that is oil soluble and a polar end that is H2O soluble. They act in essentially the same way as soap does[9]. Mixture of soap and detergent gave a more stable emulsion, increasing the charges on the oil droplets this keeps them from coalescing[8].Cholesterol and lecithin have effect on interfacial tension, but exert a proactive colloid effect reducing the potential for coalescence by providing a protective sheath around the droplets, impacting a charge to the dispersed droplets. Cholesterols give oil the capacity to absorb water; this is attributed to the little time of separation between the liquids. Lecithin on the other hand has a strong hydrophilic character[10]. When Cholesterol and lecithin were mixed, lycolecithin and cholersetyl ester were formed which are the real substances doing dissolution of oil in water[9]. When Mg(OH)2 was added it only took 1.1min for the layer between the molecules to appear again, and when Al(OH)3 was added it took 1.8min to separate. This is an indication that the two emulsifiers are poor in dissolution character. However Al(OH)3 is a better emulsifier than Mg(OH)2.Flocculation and resultant creaming represent potential steps towards complete coalescence of the dispersed phase. In addition the creaming volume is indicative of the stability of internal aqueous droplets entrapped in the emulsifier droplet, since swelling or shrinkage of the internal aqueous drops directly affect the oil droplets size and hence thee creaming volume[12].Table 3 contains the result obtained when effect of surfactant HLB was tested. It can be seen from the table that the HLB values increased from test-tube 1-7. Test-tube 8 had a zero HLB because no surfactant was added. The time of separation elapsed before the first signs of phase separation was observed, and the stability was ranked from 1, (longest time) to 8, (shortest time). At low soap and detergent concentration, rapid coalescence among the inner and outer droplets to inner phase occurred, resulting in separation within a short period of time. In table 3, Test-tube number 6 took the longest time to separate the liquids thus the highest stabilized. As time of separation decreases the stability increases.
These diagrams symbolized the two important features of the structure. The circle represents the ionic carboxylates end of the molecule and the long time represents the non-polar hydrocarbon chain. The non-polar hydrocarbon group is not soluble in water, but water would be attracted to the ionic groups and hence tend to dissolve the molecules. As soon as the soap molecules are added, the hydrocarbon portions will not permit them to be exposed to water. Instead, they are attracted to each other, forming a cluster in which they are literally dissolvedin each other. This grouping allows the ionic groups at the ends of the soap molecules to be attracted to the surrounding water molecules. The result is that the soap molecules are, in a sense, able to dissolve in the water.The agitation helps jar the oil from the surface and disperse it into tiny droplets. More and more soap molecules surround the oil, until it becomes incorporated within a soap micelle. An emulsion is achieved at this level.In table 1, it took soap 3.3mins to separate water and oil, while detergent took 3.2mins and their mixture 6.5mins this indicates that detergents are better emulsifiers than soap and their mixture more better emulsifiers. Cholesterol proved better emulsifying agents than lecithin and their mixture more stronger emulsifiers. Mg(OH)2 is a less powerful emulsifying agent than Al(OH)3. Detergent on the other hand has molecules with features they share with soap. They are amphipathic and have a large non- polar hydrocarbon end that is oil soluble and a polar end that is H2O soluble. They act in essentially the same way as soap does[9]. Mixture of soap and detergent gave a more stable emulsion, increasing the charges on the oil droplets this keeps them from coalescing[8].Cholesterol and lecithin have effect on interfacial tension, but exert a proactive colloid effect reducing the potential for coalescence by providing a protective sheath around the droplets, impacting a charge to the dispersed droplets. Cholesterols give oil the capacity to absorb water; this is attributed to the little time of separation between the liquids. Lecithin on the other hand has a strong hydrophilic character[10]. When Cholesterol and lecithin were mixed, lycolecithin and cholersetyl ester were formed which are the real substances doing dissolution of oil in water[9]. When Mg(OH)2 was added it only took 1.1min for the layer between the molecules to appear again, and when Al(OH)3 was added it took 1.8min to separate. This is an indication that the two emulsifiers are poor in dissolution character. However Al(OH)3 is a better emulsifier than Mg(OH)2.Flocculation and resultant creaming represent potential steps towards complete coalescence of the dispersed phase. In addition the creaming volume is indicative of the stability of internal aqueous droplets entrapped in the emulsifier droplet, since swelling or shrinkage of the internal aqueous drops directly affect the oil droplets size and hence thee creaming volume[12].Table 3 contains the result obtained when effect of surfactant HLB was tested. It can be seen from the table that the HLB values increased from test-tube 1-7. Test-tube 8 had a zero HLB because no surfactant was added. The time of separation elapsed before the first signs of phase separation was observed, and the stability was ranked from 1, (longest time) to 8, (shortest time). At low soap and detergent concentration, rapid coalescence among the inner and outer droplets to inner phase occurred, resulting in separation within a short period of time. In table 3, Test-tube number 6 took the longest time to separate the liquids thus the highest stabilized. As time of separation decreases the stability increases.



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


