-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2013; 3(3): 64-68
doi:10.5923/j.ijmc.20130303.04
The Use of Eco-Friendly Leaf as a Corrosion Inhibitor of Mild Steel in an Acidic Environment
Francis O. Nwosu1, Lebe A. Nnanna1, E. Osarolube2
1Department of Physics/Electronics, Abia State Polytechnic, Aba Nigeria
2Department of Physics, University of Port Harcourt, Choba Nigeria
Correspondence to: Francis O. Nwosu, Department of Physics/Electronics, Abia State Polytechnic, Aba Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The leaf extract of Achyranthes aspera L was investigated for corrosion inhibition of mild steel in 1.0M HCl medium at room temperature. Gravimetric technique was used to study the corrosion behavior in the absence and presence of different concentrations of the leaf extract. The result obtained showed that the Achyranthes aspera L is a good inhibitor for mild steel in 1.0M HCl solution. The corrosion rate and inhibition efficiency were calculated. The inhibition efficiency calculated showed a great percentage result with optimum value of 82.3%. Achyranthes aspera L extract was adsorbed on the mild steel surface in accordance with Langmuir, Frumkim, and Flory-Huggins adsorption isotherm models. The negative adsorption energy ΔGads obtained inferred that the adsorption rates were spontaneous and the interaction between the inhibitive molecules was found to be repulsive.
Keywords: AchyranthesasperaL, Corrosion Rate, Inhibition Efficiency and Adsorption Isotherm Mechanism
Cite this paper: Francis O. Nwosu, Lebe A. Nnanna, E. Osarolube, The Use of Eco-Friendly Leaf as a Corrosion Inhibitor of Mild Steel in an Acidic Environment, International Journal of Materials and Chemistry, Vol. 3 No. 3, 2013, pp. 64-68. doi: 10.5923/j.ijmc.20130303.04.
Article Outline
1. Introduction
- Corrosion phenomena, control and prevention are unavoidable major scientific issues that must be addressed daily as far as there are increasing needs of metallic materials in all facets of technological development.[1] Mild steel, a structural material is applied in many areas some of which includes reaction vessels, pipes, tanks, et cetera which are known to corrode invariably in contact with various solvents. On the other hand, acid solutions are often used in industry for cleaning, descaling and pickling of metallic structures, processes which are normally accompanied by considerable dissolution of the metal. From the point of view of nation’s economy, financial implications and hazards, it is necessary to adopt appropriate means and ways to reduce the losses due to corrosion and some of the measures employed(include anodic and cathodic protection, lubrication, painting and electroplating) have been adopted. However, one of the best options available for the protection of metals against corrosion involves the use of inhibitors[2]. Coupling to the hazardous nature of corrosion, most of the corrosion inhibitors are synthetic chemicals, expensive and very hazardous to environment. Therefore, it is desirable to source for environmentally safe, economical, green inhibitors[3][4] [5][6][7][8][9]. As a result, there has been a wide spread research on the use of plant extracts and their isolates as corrosion.[10] reported that some researchers have worked on some naturally occurring materials such as Gum Arabic, Raphia, hookeri, Ipomoea Invaculcerata, Vigna unguiculata, Pachylobus edulis, Ginseng root, Dacroydes edulis, Zenthoxylum alatum, Hisiscus sabdairffa, Datura stramonium, Limonene, prosopis, to mention but a few; and they were found to be good corrosion inhibitors. Therefore, the present study aims at investigating the inhibitive effect and adsorption properties of Achyranthes aspera L (Aa) leaves extract on mild steel corrosion in 1.0 M Hydrochloric acid using gravimetric techniques.Aa is commonly called devil’s horsewhip. It is a vascular plant widely found in temperate region. It belongs to the family of Amaranthacae and has medicinal properties used on tetanus, snake bits, treatment. Traditionally, the plant is used in asthma and cough. It is pungent, antiphlegmatic, antiperiodic, diuretic, purgative and laxative, useful in oedema, dropsy and piles, boils and eruptions of skin et cetera[11]
2. Materials and Methods
2.1. Mild Steel Preparation
- The mild steel used in the studies was analyzed using optical emission spectrometry and consists (in % weight): C(0.0285), Si(0.0096), P(0.0096), Mn(0.1965), Ni(0.0153), Cr(0.0124), Mo(0.0027), Cu(0.0137), Sn(0.043), W(0.0052), Zn(0.031), As(0.0037), Ru(0.0028), and Fe(99.657%). The metal sheet was cut into sample with the following dimensions of 30 x 30 x 1 mm and used for corrosion studies.
2.2. Preparation of A. aspera L. (devil’s horsewhip) Extract
- The extract was prepared according to[12]. Aa leaves were collected from Federal University of Technology Owerri (FUTO) in Imo State, Nigeria. They were washed with plenty water, air dried and ground to powder form. The extraction was done in a reflux setup for 3 hrs at a constant temperature of 75°C using 10g of air dried devils horsewhip in 300 ml of 1.0 M HCl solution. The solution was cooled under atmospheric pressure. The filtrate measured. Different concentrations of the inhibitor were prepared from the filtrate and the corrosive environment in the range 100, 150, 200, 250 and 300 g/L.
2.3. Gravimetric Technique
- Gravimetric technique used was according to the description by[13]. All reagents used were BDH grade. Prior to measurement, each sample was degreased in ethanol, the surface smoothened using sic emery paper (of grades 400, 600, 800 and 1000) and then double washed with distilled water and air dried after dipping in acetone. The samples were weighed using FA2104A analytical electronic digital weighing balance (sensitivity of 0.0001). The specimen were immersed in 250 ml beaker containing 240 ml of 1.0 M HCl solution of different concentrations (0, 100, 150, 200, 250 and 300 g/L) of the prepared inhibitor at room temperature (303°K). The set up were exposed for a period of five days after which the specimen were taken out, washed, dried and weighed accurately. Triplicate experiments were performed in each case and the mean value reported.
3. Results and Discussion
- The mass losses of mild steel sample in 1.0 M HCl solution, with or without different concentrations of the investigated inhibitor, were recorded after 5 days of immersion at room temperature. The corrosion rate (CR) of mild steel, inhibition efficiency and surface coverage of Aa form the weight lost measurement were calculated using the Equations 1, 2 and 3 respectively
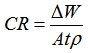 | (1) |
 | (2) |
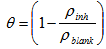 | (3) |
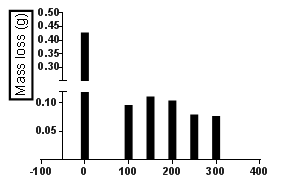 | Figure 1. Mass loss for mild steel in the difference concentration of Aa at toom temperature exposured for 5days |
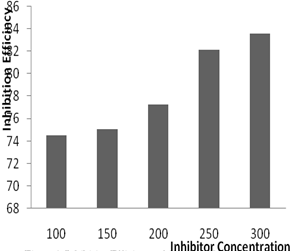 | Figure 2. Inhibition Efficiency of Aa on mild steel in 1.0 MHCl solution |
3.1. Adsorption Mechanism
- Adsorption isotherm is necessary in studying the mechanism of adsorption and also the adsorption characteristics of the inhibitor. Studies have shown that the inhibition of metal corrosion by organic compounds is attributed to either the adsorption of inhibitor molecule or the formation of a layer of insoluble complex of the metal on the surface which acts as a barrier between the metal surface and the corrosive medium[14]. The study found that there is no insoluble material on the metal surface, and this suggests that the inhibitive action of the Aa leave extract may be due to its adsorption on the metal surface.In accounting for the observed protective effect, it should be noted that experiments was carried out in biochemistry laboratory, Abia State Polytechnic, Aba, Nigeria shows that the extract comprise mixture of organic and resinous matter (sapogenins, alchorneine, alchorneinone, alkaloids, anthranilic acid, gentisinic acid yohimbine, flavonoids, glycosides) some of which have good corrosion inhibiting abilities. The complex chemical compositions make it rather difficult to assign the inhibiting action to a particular constituent or group of constituents. Nevertheless, the net adsorption of the extract organic matter on the metal surface creates a barrier to charge and mass transfer, thus protecting the mild steel surface from corrodent attack[15]. The degree of protection varies for different extracts, with notable sensitivity to the inhibitor concentration.[10] reported that leaf extract from Chlomolaena odorata L. (LECO) contained similar constituents and the leaf extract showed a good inhibitive action against the corrosion of Al in 2 M HCl.However,[16] accounted that the relationship between inhibition efficiency and the bulk concentration of the inhibitor at constant temperature is known as isotherm; thus the following insight into the adsorption process. Several adsorption isotherm were attempted to fit surface coverage values to classical isotherm of Langmuir, Temkin, Frumkin, Flory-Huggins[17]. Furthermore, the value of the correlation (R2) was used to determine the best fit isotherm which was obtained for Langmuir, Frumkin isotherms and Flory-Huggins.
3.2. Langmuir Adsorption Isotherms
- Langmuir adsorption isotherm is calculated from the experimental data and can be expressed according to Equation 4[18]
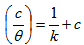 | (4) |
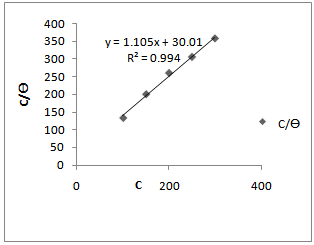 | Figure 3. Langamiur adsorption isotherm for Aa in 1.0 M HCl |
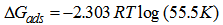 | (5) |
3.3. Frukim Adsorption Isotherm
- Frumkin isotherm is an extension of Langmuir isotherm. It states that adsorbed molecules do interact and affect further adsorption by either repulsion or attraction of moleculesThe Frumkin isotherm widely used to quantify the interactions occurring between corrosion inhibitor and a metal surface[22][23][24], and it is expressed by the equation 6[23]
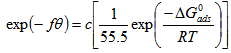 | (6) |
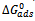 is the standard free energy of adsorption (kJ/mol). The Frumkin adsorption isotherm is a general expression since the limiting case for which f = 0 is representative of an interaction free behavior between adsorbed species and defines the Langmuir isotherm[28][23]. Equation 6 can be rearranged to give equation 7,[23].
is the standard free energy of adsorption (kJ/mol). The Frumkin adsorption isotherm is a general expression since the limiting case for which f = 0 is representative of an interaction free behavior between adsorbed species and defines the Langmuir isotherm[28][23]. Equation 6 can be rearranged to give equation 7,[23].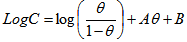 | (7) |
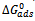 /2.3RT) + log 55.5 and has the meaning of equilibrium constant of the adsorption process. Equation 7 was used to plot the Frumkin isotherm (Ө against Log C) shown in Figure 4. The existence of adsorption interactions between adsorbed Aa extract and the metal surface is thus confirmed since most of the experimental data fit nicely into the Frumkin isotherm plot, the slight S-shape. The adsorption parameter f was -5.56 using two best values of C (100 – 300 g/L) and the corresponding Ө. The negative value of f indicates that the adsorption of the tested compound is accompanied by mutual repulsion of the inhibitor molecules,[17]
/2.3RT) + log 55.5 and has the meaning of equilibrium constant of the adsorption process. Equation 7 was used to plot the Frumkin isotherm (Ө against Log C) shown in Figure 4. The existence of adsorption interactions between adsorbed Aa extract and the metal surface is thus confirmed since most of the experimental data fit nicely into the Frumkin isotherm plot, the slight S-shape. The adsorption parameter f was -5.56 using two best values of C (100 – 300 g/L) and the corresponding Ө. The negative value of f indicates that the adsorption of the tested compound is accompanied by mutual repulsion of the inhibitor molecules,[17]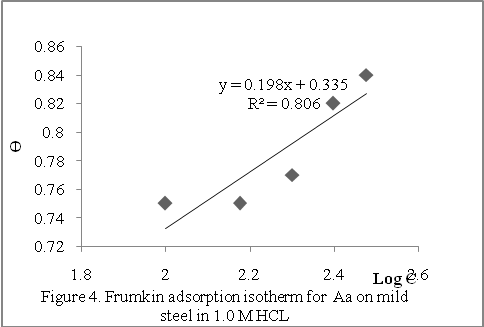 | Figure 4. Frumking adsorption isotherm for Aa on mild steel in 1.0 MHCL |
3.4. Flory-Huggins Isotherm
- The assumptions of the Flory-Huggins adsorption isotherm can be expressed according to equation 8 as reported by[25]
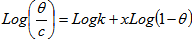 | (8) |
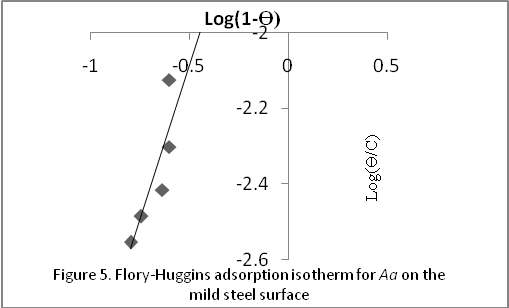 | Figure 5. Flory-Huggins adsorption isotherm for Aa on mild steel surface |
4. Conclusions
- The present study found that Aa is a good corrosion inhibitor of mild steel in 1.0 M HCl medium at room temperature. Aa leaf extract significantly reduced the corrosion rate of mild steel in the acidic medium and it was found that the reduction rate of corrosion occurred with increase in the inhibitor concentration.Given this inhibition efficiency, the value of Gibb’s free energy of adsorption indicate that Aa leaf extract is physically adsorbed on the surface and spontaneous following its correspondence to the following adsorption models: the Langmuir, Frumkin and Flory-Huggins. The interactions of the adsorbed molecules of the inhibitor are repulsive and bulky on the metal surface.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML