-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2013; 3(2): 17-27
doi:10.5923/j.ijmc.20130302.01
“N,N-Bis(phosphonomethyl) Glycine, Zn2+ and Tartrate” – A New Ternary Inhibitor Formulation for Corrosion Control of Carbon Steel
B. V. Appa Rao1, M. Venkateswara Rao2, S. Srinivasa Rao3, B. Sreedhar4
1Department of Chemistry, National Institute of Technology, Warangal, 506004, India
2Department of Chemistry, Andhra Loyola College, Vijayawada, 520008, India
3Department of Chemistry, V. R. Siddhartha Engineering College, Vijayawada, 520007, India
4Inorganic and Physical Chemistry Division, Indian Institute of Chemical Technology, Hyderabad, 500007, India
Correspondence to: S. Srinivasa Rao, Department of Chemistry, V. R. Siddhartha Engineering College, Vijayawada, 520007, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Studies on inhibition of corrosion of carbon steel in aqueous environment using N,N-Bis(phosphonomethyl) glycine (BPMG), zinc ions and tartrate are presented. The investigations revealed that tartrate acts as an excellent synergist in corrosion inhibition. Optimum concentrations of all the three components of the ternary formulation are established by gravimetric studies. Potentiodynamic polarization studies indicate that the new ternary system is a mixed inhibitor. Results of the impedance studies show that a protective film is formed on the metal surface in presence of the inhibitor formulation. Analysis of the protective film using X-ray photoelectron spectroscopy (XPS) and reflection absorption Fourier transform infrared (FTIR) spectroscopy infer the presence of Zn(OH)2, oxides and hydroxides of iron and the inhibitor molecules in the surface film probably in the form of a complex, [Zn(II)-BPMG-tartrate]. A plausible mechanism of corrosion inhibition is proposed.
Keywords: Corrosion Inhibitor, Carbon Steel, Phosphonated Glycine, Tartaric Acid, Synergism
Cite this paper: B. V. Appa Rao, M. Venkateswara Rao, S. Srinivasa Rao, B. Sreedhar, “N,N-Bis(phosphonomethyl) Glycine, Zn2+ and Tartrate” – A New Ternary Inhibitor Formulation for Corrosion Control of Carbon Steel, International Journal of Materials and Chemistry, Vol. 3 No. 2, 2013, pp. 17-27. doi: 10.5923/j.ijmc.20130302.01.
Article Outline
1. Introduction
- Carbon steel is an important material of construction for cooling water systems and heat exchangers in industries. In order to control corrosion of carbon steel in such systems, application of corrosion inhibitors is an effective practice. After the exit of formulations based on chromates, phosphates, etc., phosphonate based formulations are introduced and they have been in use as corrosion inhibitors since the past three decades[1-8]. Phosphonates in combination with zinc ions proved to be effective corrosion inhibitors for carbon steel[1-8]. However, they require higher concentrations of both phosphonate and zinc ions for an effective inhibition. An efficient technique to reduce the concentrations of both phosphonate and toxic zinc ions in the inhibitor formulations is to add a non-toxic organic/inorganic compound as a synergist. A few of such ternary inhibitor formulations containing phosphonate, zincions and organic/inorganic additive were reported in literature[9-13]. These reports suggest that the high inhibition efficiency exhibited by the ternary formulations is due to synergism existing among the inhibitor components of the formulations. In this background, the present study was carried out using an environmentally friendly organic compound namely tartaric acid as a synergist to the binary formulation containing N,N-Bis(phosphonomethyl) glycine (BPMG) and zinc ions for corrosion control of carbon steel. It was reported in a previous study that BPMG-Zn2+ binary formulation acts as an effective corrosion inhibitor for carbon steel[8]. Tartaric acid is chosen as the synergist for the present study due to presence of two carboxyl groups and two hydroxyl groups in its structure, which provide complexing ability with metal ions. The main objective of the present study is to investigate the inhibitory effects of the new ternary inhibitor formulation containing BPMG, Zn2+ and tartrate in corrosion inhibition of carbon steel in low chloride aqueous environment. Also, it is of interest to study the synergistic effect of tartrate in combination with BPMG and Zn2+ on corrosion inhibition. For the present study, 200 ppm of NaCl solution has been chosen as control because of the following reason. The water used in cooling water systems is generally either demineralized water or unpolluted surface water. In either case, the aggressiveness of the water will never exceed that of 200 ppm of NaCl.
2. Experimental Section
2.1. Solutions and Specimens
- For all the studies, the specimens taken from a single sheet of carbon steel of the following composition were chosen. C – 0.1 to 0.2 %, P – 0.03 to 0.08 %, S – 0.02 to 0.03%, Mn – 0.4 to 0.5 % and the rest iron. Prior to the tests, the specimens were polished to mirror finish with 1/0, 2/0, 3/0 and 4/0 emery polishing papers respectively, washed with distilled water, degreased with acetone and dried. For gravimetric measurements, the polished specimens of the dimensions, 3.5 cm x 1.5 cm x 0.2 cm, were used while for other studies, the dimensions of the specimens are 1.0 cm x 1.0 cm x 0.1 cm. BPMG (C4H11P2O8N) obtained from Aldrich Chemical Company Inc., USA was used in the present study. Zinc sulphate (ZnSO4.7H2O), potassium sodium tartrate tetrahydrate (C4H4NaKO6.4H2O) and other reagents were analytical grade chemicals. All the solutions were prepared by using triple distilled water. The pH values of the solutions were adjusted by using 0.01 N NaOH and 0.01 N H2SO4 solutions. An aqueous solution consisting of 200 ppm of NaCl has been used as the control throughout the study.
2.2. Gravimetric Measurements
- In all the gravimetric experiments, the polished specimens were weighed and immersed in duplicate, in 100 mL control solution in the absence and presence of inhibitor formulations of different concentrations, for a period of seven days. Then the specimens were reweighed after washing, degreasing and drying. During the studies, only those results were taken into consideration, in which the difference in the weight-loss of the two specimens immersed in the same solution did not exceed 0.1 mg. Accuracy in weighing upto 0.01 mg and in surface area measured upto 0.1 cm2, as recommended by ASTM G31, was followed[14]. The immersion period of seven days was fixed in view of the considerable magnitude of the corrosion rate obtained in the absence of any inhibitor after this immersion period. The immersion period was maintained accurately upto 0.1 h in view of the lengthy immersion time of 168 h. Under these conditions of accuracy, the relative standard error in corrosion rate determinations is of the order of 2 % or less for an immersion time of 168 h[15]. Corrosion rates (CR) of carbon steel in the absence and presence of various inhibitor formulations are expressed in mmpy. Inhibition efficiencies (IEg) of the inhibitor formulations were calculated by using the formula,
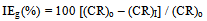 | (1) |
2.3. Electrochemical Studies
- Both the potentiodynamic polarization studies and electrochemical impedance spectroscopic (EIS) studies were carried out using the Electrochemical Workstation Model IM6e, Zahner-elektrik, GmbH, Germany and the experimental data were analyzed by using the Thales software. The measurements were conducted in a conventional three electrode cylindrical glass cell with platinum electrode as auxiliary electrode and saturated calomel electrode (SCE) as reference electrode. The working electrode was carbon steel embedded in epoxy resin of polytetrafluoroethylene so that the flat surface of 1 cm2 was the only surface exposed to the electrolyte. The three-electrode set up was immersed in control solution of volume 500 mL both in the absence and presence of various inhibitor formulations and allowed to attain a stable open circuit potential (OCP). The pH values of the solutions were adjusted to 7.0 and the solutions were unstirred during the experiments.Polarization curves were recorded in the potential range of –750 to –150 mV with a resolution of 2 mV. The curves were recorded in the dynamic scan mode with a scan rate of 2 mV s-1 in the current range of –20 mA to +20 mA. The ohmic drop compensation has been made during the studies. The corrosion potential (Ecorr), corrosion current (Icorr), anodic Tafel slope (βa) and cathodic Tafel slope (βc) were obtained by extrapolation of anodic and cathodic regions of the Tafel plots. The inhibition efficiency (IEp) values were calculated from Icorr values using the equation[16],
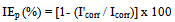 | (2) |
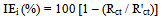 | (3) |
2.4. Surface Analysis By X-Ray Photoelectron Spectroscopy (XPS)
- XPS measurements of the surface film was carried out with Kratos analytical photoelectron spectrometer (AXIS 165) with monochromated Al Kα X-ray source (1486.6 eV) operated at 100 W. The spectra were collected at an electron take-off angle of 90o. Analyser pass energy was 80 eV, with a step of 1 eV. Deconvolution spectra were recorded with analyser pass energy of 80 eV, with a step of 0.1 eV for the elements of interest namely Fe 2p, O 1s, P 2p, C 1s, N 1s and Zn 2p. Binding energies for the deconvolution spectra were corrected individually for each measurement set, based on a value of 285.0 eV for the C–C component of C 1s.
2.5. Fourier Transform Infrared (FTIR) Spectroscopic Studies
- FTIR spectra were recorded using FTIR spectrophotometer from Thermo Electron Corporation, USA, model Nexus 670 with a resolving power of 0.125 cm-1. The detector is temperature stabilised DTGS (KBr window) and liquid nitrogen cooled MCT-A and the beam splitter is XT-KBr. FTIR spectra of pure BPMG and pure potassium sodium tartrate were recorded using the KBr pellet method. The reflection absorption FTIR spectra of the surface films were recorded in the wave number range of 4000–400 cm-1. The measurements were made at a grazing angle of 85o.
3. Results and Discussion
3.1. Gravimetric Measurements
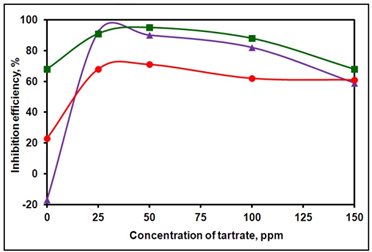
- Figure 1 shows the inhibition efficiency of the ternary system, BPMG-Zn2+-tartrate, as a function of concentration of tartrate at different concentrations of BPMG and Zn2+. From the figure, it can be observed that in case of all these ternary formulations at pH 7, as the concentration of tartrate is increased, the inhibition efficiency increases, reaches a maximum at an optimum concentration of tartrate and then decreases. In order to achieve an inhibition efficiency > 90 %, the required minimum concentrations of BPMG and Zn2+ are 20 ppm and 30 ppm respectively in presence of tartrate. While the binary system consisting of 20 ppm BPMG and 30 ppm Zn2+ accelerates corrosion, with the addition of just 25 ppm of tartrate, the inhibition efficiency of the ternary formulation is 92 %. Further increase in the concentration of tartrate ions reduced the inhibition efficiency reaching 59 % at 150 ppm of tartrate. In case of the binary system containing 30 ppm each of BPMG and Zn2+, the inhibition efficiency is 68 %, which is increased to 95 % by the addition of 50 ppm of tartrate. On further increase in the concentration of tartrate, say to 100 ppm and 150 ppm, the inhibition efficiency is reduced to 88 % and 68 % respectively. From these results, it can be inferred that in case of the ternary system consisting of BPMG, Zn2+ and tartrate, the formulation containing optimum concentration of each of the components gives the highest inhibition efficiency. In other words, optimum amounts of each of the three components must be adsorbed on the surface of the metal, so that each one of them plays its own role in the formation of protective film either through complex formation or through formation of Zn(OH)2 on cathodic sites, covering the entire metal surface. At higher concentrations of tartrate ions viz., 100 ppm and above in the bulk of the solution, more tartrate is reaching the surface of the metal at the cost of the required optimum concentrations of BPMG and Zn2+ on the surface and therefore the necessary optimum concentration of BPMG and Zn2+ required for the formation of protective film, is not available on the surface. Hence, the decrease in inhibition efficiency is observed at concentrations of tartrate ions ≥ 100 ppm. It may be mentioned here that the molar ratio of BPMG : Zn2+ : tartrate is 1.0 : 5.4 : 2.0 to exhibit excellent synergism. The role of each synergist is explained under mechanistic aspects of corrosion inhibition.
|
3.2. Potentiodynamic Polarization Studies
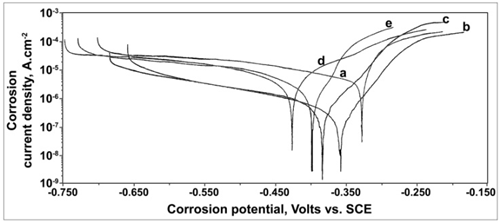
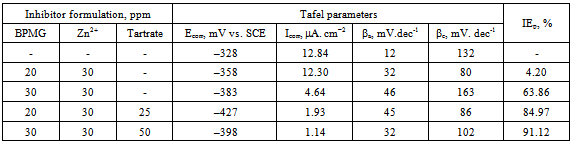
- The potentiodynamic polarization curves of carbon steel electrode in 200 ppm of chloride solution at pH 7 in the absence and presence of various inhibitor formulations are shown in Figure 3. The corrosion parameters are listed in Table 2. The Tafel potential (Ecorr) in case of the control solution is –328 mV vs. SCE. It is shifted to more cathodic side in case of all the inhibitor formulations considered for polarization studies. The corrosion current density (Icorr) in case of the control solution is 12.84 μA.cm–2. In presence of all the inhibitor systems, the decrease in corrosion current is observed. The significant reduction in Icorr value in presence of the ternary inhibitor formulations indicates the decrease in corrosion rate in presence of the ternary inhibitor systems.An examination of the Tafel slopes reveals that the addition of 20 ppm of BPMG, 30 ppm of Zn2+ and 25 ppm of tartrate to the control solution shifts the anodic Tafel slope to an extent of 33 mV.dec-1, while the shift in cathodic Tafel slope is 46 mV.dec-1. Similarly, in presence of 30 ppm of BPMG, 30 ppm of Zn2+ and 50 ppm of tartrate, the shift in anodic Tafel slope is 20 mV.dec-1 and that in cathodic Tafel slope is 30 mV.dec-1. These shifts indicate that the ternary inhibitor system under study retards both the anodic and cathodic partial reactions of the corrosion process and hence act as mixed type inhibitor. This is in agreement with several reports of literature indicating that phosphonate-based inhibitor formulations are mixed type inhibitors. Pech-Canul and Chi-Canul[17] investigated the inhibitive effect of N-phosphonomethyl glycine (NPMG) on the corrosion of carbon steel in neutral solutions using electrochemical techniques. From the results of polarization studies, they inferred that NPMG-Zn2+ system acts as a mixed type inhibitor.
|
3.3. Electrochemical Impedance Studies
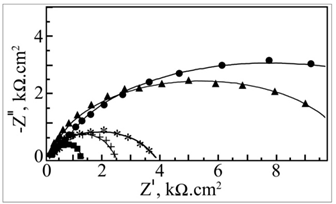
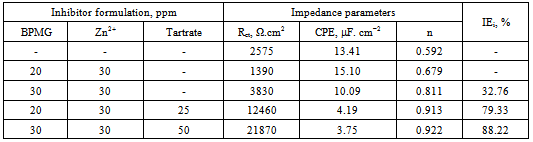
- The impedance spectra in the form of Nyquist plots for carbon steel immersed in various environments at pH 7 are given in Figure 4. These plots are discussed to throw light on the nature of the protective film. The plots are depressed semicircles with the centre below the real axis. This kind of phenomenon is known as dispersing effect[18]. When the complex plane impedance contains a depressed semicircle with the centre below the real axis, which is characteristic for solid electrodes, it is often attributed to roughness and inhomogeneities of the solid surface[19]. It is also attributed to the distribution of active sites, adsorption of inhibitor molecules and formation of porous layers[20]. In such cases, the parallel network charge transfer resistance -double layer capacitance (Rct-Cdl) is a poor approximation especially for systems where an efficient inhibitor is present. Considering that the impedance of a double layer did not behave as an ideal capacitor in presence of the dispersing effect, a constant phase element (CPE) is substituted for the capacitor to fit the depressed semicircles more exactly[21]. The admittance and impedance of a CPE are, respectively defined asYCPE = Yo (jω)n andZCPE = A (jω)-n, where Yo is the modulus, ω is the angular frequency, n is the CPE exponent and A is a proportional factor mathematically the reciprocal of modulus[22,23]. For a highly polished electrode, the value of n is close to 1.0. The lower the value of n, the rougher is the electrode. It can be seen that when n = 1, the element CPE is an ideal capacitor and A is equal to the capacitance, C. Figure 4 indicates that the Nyquist plots are characterized by a single time constant. The experimental data obtained from these plots are fitted by the equivalent electrical circuit shown in Figure 5. Such an equivalent circuit was also discussed by several authors[22,23], who obtained similar depressed semicircles with a single time constant. The impedance parameters obtained from Nyquist plots and the inhibition efficiencies (IEi) calculated from these parameters are shown in Table 3.
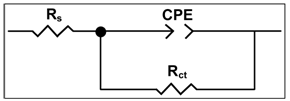 | Figure 5. Equivalent circuit used to fit the impedance data |
|
3.4. Interpretation of X-Ray Photoelectron Spectra
- The X-ray photoelectron spectrum of the surface film formed on carbon steel immersed in the environment containing BPMG (20 ppm) + Zn2+ (30 ppm) + tartrate (25 ppm) is shown in Figure 6. The XPS spectrum shows binding energy peaks due to electrons of various elements present in the surface film. The corresponding computer deconvolution spectra of the individual elements are shown in Figures 7 and 8. The interpretation of all the spectra is done with the help of the data of the elemental binding energies reported in literature and also with the help of reports published on the analysis of XPS spectra of the surface films formed on carbon steel.
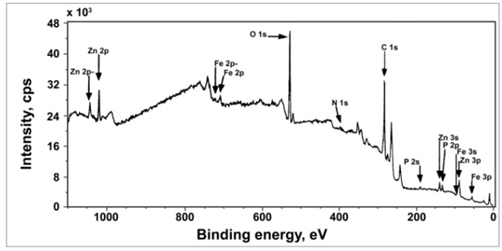 | Figure 6. XPS survey spectrum of the surface film formed in presence of the ternary inhibitor formulation |
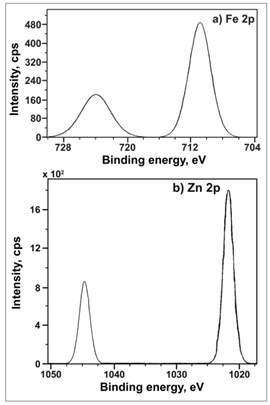 | Figure 7. XPS deconvolution spectra of the elements in the surface film, a) Fe 2p and b) Zn 2p |
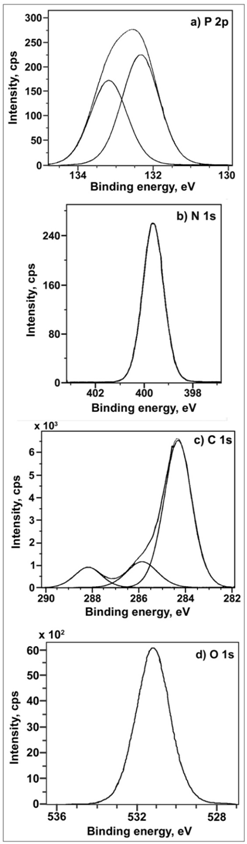 | Figure 8. XPS deconvolution spectra of the elements in the surface film, a) P 2p, b) N 1s, c) C 1s and d) O 1s |
3.5. Interpretation FTIR Spectra
- The reflection absorption FTIR spectrum of the surface film formed on carbon steel in presence of the ternary inhibitor formulation, BPMG (20 ppm) + Zn2+ (30 ppm) + tartrate (25 ppm), is shown in Figure 9. This spectrum is compared with the FTIR spectra of pure BPMG and pure potassium sodium tartrate tetrahydrate in KBr pellet. The spectra of pure compounds are not included in this paper. The reflection absorption FTIR spectrum of the surface film can be interpreted as follows. The peak due to P–OH stretching vibrations is observed at 1140 cm-1. This result can be interpreted due to interaction of free P – O- present in the phosphonate with metallic species, viz., Fe(III) and Zn(II) to form P–O–Metal bonds[4,36]. Raman et al.[37] reported that a peak due to P–OH stretching vibrations appear in the range of 1100-1200 cm-1[38]. It infers that there is presence of BPMG in the surface film. The two peaks appeared at 1680 cm-1 and 1520 cm-1 in the spectrum of the surface film may be due to the carbonyl group of BPMG and due to carbonyl group of tartrate ions. These are shifted from 1732 cm-1 and 1740 cm-1 observed for carbonyl group of BPMG and cabonyl group of tartrate ion respectively in the spectra of pure compounds. The two peaks due to carbonyl groups in the spectrum of the surface film are observed to be at lower frequencies when compared to the corresponding peaks of pure compounds. It is therefore inferred that BPMG and tartrate ion are involved in complex formation with Fe(III) as well as Zn(II) and the presence of [Fe(III), Zn(II)–BPMG–tartrate] hetero-polynuclear complex is suggested. The small peak observed at 1320 cm-1 is due to the presence of Zn(OH)2[39]. A broad band appeared around 3280 cm-1 can be interpreted due to O–H stretching vibrations[40]. The hydroxyl group is present in BPMG, tartrate ions, Zn(OH)2 and Fe(OH)3. Hence this peak supports the presence of these compounds within the protective film. The peak at 2360 cm-1 can be attributed to strong hydrogen bond, due to which the tartrate exists as a dimer or trimer[41]. In case of pure potassium sodium tartrate, this peak is observed at 2500 cm-1. The peaks observed at 570 cm-1 and 630 cm-1 in the spectrum of the surface film can be assigned to amorphous oxides of Fe2O3 and Fe3O4[42]. From the reflection absorption FTIR spectrum of the surface film, it is inferred that apart from [Fe(III),Zn(II)–BPMG–tartrate] hetero-polynuclear complex, the protective film consists of Zn(OH)2 and oxides and hydroxides of iron (III).
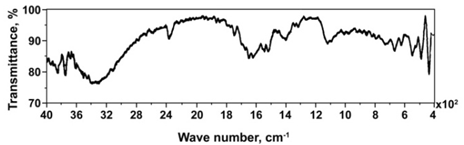 | Figure 9. Reflection absorption FTIR spectrum of the surface film formed in presence of the ternary inhibitor formulation |
3.6. Mechanism of Corrosion Inhibition
- In order to explain all the experimental results, a plausible mechanism of corrosion inhibition is proposed as follows:The mechanism of corrosion of carbon steel in nearly neutral aqueous media is well established. The well-known reactions are mentioned below.
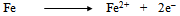 | (4) |
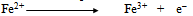 | (5) |
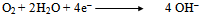 | (6) |
 | (7) |
 | (8) |
4. Conclusions
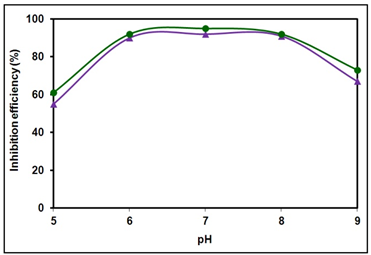
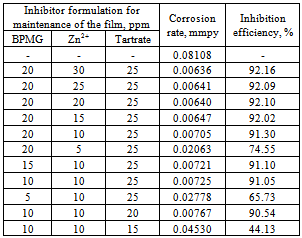
- Tartaric acid, a non-toxic organic compound is proved to be an excellent synergist in combination with BPMG and Zn2+ for corrosion control of carbon steel in nearly neutral aqueous environment. The ternary formulation containing 20 ppm of BPMG and 30 ppm of Zn2+ along with 25 ppm of tartrate is an effective corrosion inhibitor for carbon steel. Once the protective film is formed, a mixture of only 10 ppm each of BPMG and Zn2+ and 20 ppm of tartrate will serve as the maintenance dosage. The ternary inhibitor system is thus relatively more environmentally friendly. The inhibitor system is effective in the pH range 6-8. The inhibitor formulation acts as a mixed type inhibitor. Electrochemical impedance studies indicated the significant modification of the metal/solution interface by the formation of dense protective film in presence of the inhibitor formulation. The protective film consists of mainly [Zn(II)–BPMG–tartrate] complex, Zn(OH)2 and small amounts of oxides/hydroxides of Fe(III). Presence of optimum amounts of all these compounds is required at a given pH value to make the surface film protective.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML