-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2013; 3(1): 10-16
doi:10.5923/j.ijmc.20130301.03
Adsorption and Corrosion Inhibtion of Gnetum africana Leaves Extract on Carbon Steel
Lebe A. Nnanna1, Israel O. Owate2, Onyinyechi C. Nwadiuko1, Nneka D. Ekekwe3, Wisdom J. Oji1
1Department of Physics/Electronics, Abia State Polytechnic, PMB 7166, Aba, Nigeria
2Department of Physics, University of Port Harcourt, PMB 5323, Port Harcourt, Nigeria
3Department of Chemistry, Abia State Polytechnic, PMB 7166, Aba, Nigeria
Correspondence to: Lebe A. Nnanna, Department of Physics/Electronics, Abia State Polytechnic, PMB 7166, Aba, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Adsorption of Gnetum africana leaves extract and corrosion inhibition of carbon steel in hydrochloric acid solutions have been investigated using gravimetric technique. Inhibition efficiency increased with extract concentration and time of exposure. The effect of temperature on the corrosion behavior of mild steel in 1.0 M HCl with addition of plant extracts was studied at the temperature range of 303–333±1K. Inhibition efficiency of 92.42% was obtained. An adsorption mechanism involving physisorption and chemisorptions of extract constituents at low and high temperatures, respectively, has been proposed from the trend of adsorption free energies. The experimental data complied to the Langmuir and Temkin adsorption isotherms and the negative values of the Gibb’s free energy of adsorption obtained suggested that inhibitor molecules have been spontaneously adsorbed onto the C-steel.
Keywords: Adsorption Isotherm, Corrosion Inhibition, Gnetum Africana, Free Energy of Adsorption
Cite this paper: Lebe A. Nnanna, Israel O. Owate, Onyinyechi C. Nwadiuko, Nneka D. Ekekwe, Wisdom J. Oji, Adsorption and Corrosion Inhibtion of Gnetum africana Leaves Extract on Carbon Steel, International Journal of Materials and Chemistry, Vol. 3 No. 1, 2013, pp. 10-16. doi: 10.5923/j.ijmc.20130301.03.
Article Outline
1. Introduction
- The use of inhibitors is one of the most practical methods for protecting metals against corrosion, especially in acidic media[1]. Acid solutions are widely used in industry: some of the important fields of application are acid pickling of steel, chemical cleaning and processing, ore production and oil well acidizing. As ordinary acids, HCl and H2SO4 are usually used as industrial acids, cleaning and pickling acids. Due to the general aggression of acid solutions, inhibitors are commonly used to retard the corrosive attack on metallic materials. During past decades, some commercial inhibitors have been synthesized and used successfully to inhibit corrosion of steel in acidic media. However, the major problem associated with most of these inhibitors is that they are not eco-friendly but toxic and expensive. Therefore, the study of new non-toxic corrosion inhibitors is essential to overcome this problem. The research in the field of eco-friendly corrosion inhibitors has been addressed toward the goal of using cheap, effective compounds at low or “zero” environmental impact.Plant extracts are low-cost and biodegradable, and so the study of plant extracts as corrosion inhibitors is an important scientific research field due to both economic and environmental benefits. It has been found that certain organic substances containing polar functions with nitrogen, sulphur and/or oxygen atoms in the conjugated system have been reported to exhibit good inhibiting properties of steel in acidic and alkaline environments[2-7]. The results of parallel studies of the inhibiting effects of organic compounds suggest that the inhibitory behavior of the organic compounds subsists in some chemical species or molecules in the inhibiting substances forming a protective layer between the metal surface and the corrodents. The adsorbate layer formed isolates the metal surface from the corrodents thereby reducing the corrosion rate of the metal surface. It has been recognized that the use of organic inhibitors, particularly the naturally occurring organic inhibitors of plant origin, are viable and highly beneficial since they are essentially non-toxic, environmentally benign, readily available, renewable and inexpensive[3-5],[8-20]. Through these studies, it is agreed that the inhibition performance of plant extracts is normally ascribed to the presence, in their composition, of complex organic species such as tannins, alkaloids and nitrogen bases, carbohydrates, amino acids and proteins as well as hydrolysis products. These organic compounds contain polar functions with N, S, O atoms as well as conjugated double bonds or aromatic rings in their molecular structures, which are the major adsorption centres. In the present work, Gnetum africana leaf extract is chosen to be the corrosion inhibitor.
2. Materials and Methods
2.1. Materials Preparation
- The sheets of mild steel used for this study were obtained from Federal University of Petroleum Resources, Effurun- Warri, Nigeria. Each sheet was 1.32 mm in thickness and were mechanically pressed cut into 2 cm
 2 cm coupons. These coupons were polished mechanically with Sic papers of grade 200, 400 and 600. They were degreased in ethanol, dried in acetone and stored in moisture free desiccators before their use in corrosion studies. The weight percentage composition of the mild steel is: Si-0.051%, Cu-0.185%, Mn-1.102%, P-0.919%, Pb-0.074%, S-0.783%, Mo-0.027%, V-0.014% and the remainder being Fe was used.
2 cm coupons. These coupons were polished mechanically with Sic papers of grade 200, 400 and 600. They were degreased in ethanol, dried in acetone and stored in moisture free desiccators before their use in corrosion studies. The weight percentage composition of the mild steel is: Si-0.051%, Cu-0.185%, Mn-1.102%, P-0.919%, Pb-0.074%, S-0.783%, Mo-0.027%, V-0.014% and the remainder being Fe was used.2.2. Preparation of the leaf extracts of Gnetum africana
- The procedure for the preparation of the leaf extracts is similar to that reported recently by[21]. Gentum africana leaves were collected from Abiriba, Abia State, Nigeria. They were dried in an N53C-Genlab Laboratory oven at 50⁰C, and ground to powder form. 10 g of the powder was digested in 1 L of 1 M HCl solution. The resultant solution was kept for 24 h, filtered and stored. From the stock solution, test solutions of the leaf extracts were prepared at concentration range of 0.1 – 0.5 g/L using excess acid as solvent at room temperature and 60
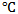 using water baths.
using water baths.2.3. Gravimetric experiment
- The cleaned and dried specimens were weighed before immersion into the respective test solutions of 1 M HCl using JA 1003A electronic weighing balance with the accuracy of
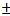 005. Tests were conducted with different concentrations of inhibitor. At the end of the tests, the specimens were carefully washed in absolute ethanol having used nitric acid to quench further corrosion from taking place, and then reweighed. Triplicate experiments were performed in each case and the mean values reported.
005. Tests were conducted with different concentrations of inhibitor. At the end of the tests, the specimens were carefully washed in absolute ethanol having used nitric acid to quench further corrosion from taking place, and then reweighed. Triplicate experiments were performed in each case and the mean values reported.3. Results and Discussion
3.1. FTIR of Gnetum africana
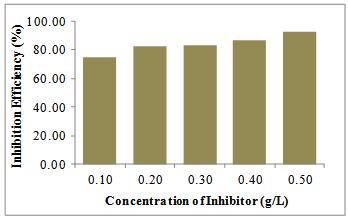
- Fourier transform infrared (FTIR) is a powerful technique that is always used to determine the type of bonding for organic inhibitors adsorbed on the metal surface[22-24]. Figure 3 shows the FTIR spectrum of the G. africana powder. The strong 3520 cm-1 is attributed to N-H or O-H stretching vibration and that at 2880 cm-1 is related to C-H stretching vibration. The strong band at 1643 cm-1 is assigned to C=C and C=O stretching vibration. Owing to the conjugation effect of flavonoids of G. africana, the C=O peak shifts from about 1700 cm-1 to lower wave number (approximately 1644 cm-1), C=C and C=O stretching vibration bands are superposition[25]. The adsorption bands at 1452 and 1122 cm-1 could be assigned to the framework vibration of aromatic ring. These results indicate that G. africana contains O and N atoms in functional groups (O-H, N-H, C=C, C=O) and aromatic ring, which meets the general structural consideration of the corrosion inhibitor.
3.2. Gravimetric technique and corrosion rates
- The corrosion rates of the mild steel in 1 M HCl solutions in the absence and presence of Gnetum africana leaf extract were determined at room temperature (303K). Figure 1 illustrates the variation of the corrosion rates of the mild steel in 1 M HCl with inhibitor concentration for an exposure time of 9 hours. Figure 1 shows clearly that the leaf extract retards the corrosion rate of the mild steel in the test solutions. The equation for corrosion rate is given by
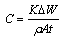 | (1) |
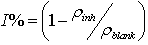 | (2) |
 | Figure 1. Corrosion rate of 1.0M HCl on mild steel in the presence of Gnetum africana against concentration of Gnetum africana after 9 hours of exposure at 30ºC |
3.3. Corrosion Inhibition and Adsorption Mechanism
- The mechanism of action of a corrosion inhibitor depends on the electron density and polarizability of the functional groups present in the molecule. Such determination of inhibition mechanisms for G. africana is complicated by the fact that most of the constituents such as alkaloids, saponins, flavonoids, phenols, tannins, sterols, terpenoids, vitamins as well as their acid hydrolysis products inhibit the corrosion reaction in different ways, which makes it rather difficult to attempt to assign the observed corrosion inhibiting effect to any particular constituent. For instance, in the acid extract of G. africana used in this study, some of the constituents may be adsorbed as protonated species and some as (non-protonated) molecular species, with the predominant adsorption mode depending on the prevailing test conditions at any time. It is noteworthy that chloride ions present in the test solutions have the tendency to be specifically adsorbed on metal surfaces, where they facilitate adsorption of protonated inhibitor species by forming intermediate bridges between the metal surfaces and the inhibitor[31]. Such protonated species are often adsorbed at cathodic sites on the metal surface and hence retard the hydrogen evolution reaction[32], which is probably responsible for the pronounced cathodic inhibiting effect of G. africana at ambient temperature (30 ºC).It is obvious from Figure 4 that corrosion rates are higher at 60ºC. This is because rise in temperature exponentially accelerates the rates of corrosion processes in media where hydrogen gas evolution accompanies corrosion, resulting in higher dissolution rates of metals. Higher rates of hydrogen gas generation increasingly agitate the metal/corrodents interface and could hinder inhibitor adsorption or perturb already adsorbed inhibitor, especially when the interaction between the metal and the inhibitor is relatively weak. As a result, the efficiency of a considerable number of organic inhibitors is significantly reduced when the temperature of the system is increased, a trend often attributed to physical rather than chemical adsorption of the inhibiting species on the corroding metal surface. Interestingly, Figure 4 clearly shows that the efficiency of G. africana at all studied concentrations improved with rise in temperature, which means that interaction between G. africana and the C-steel surface scale up significantly as temperature is increased. This is again evidence that G. africana is an effective corrosion inhibitor for C-steel in hydrochloric acid. Improvement in inhibition efficiency with increasing temperature has also been attributed to a change in the nature of adsorption, wherein the inhibitor is physically adsorbed at lower temperature whilst chemisorptions is favoured at higher temperature[2].
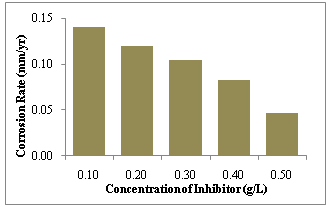
 | Figure 2. Inhibition efficiency of Gnetum africana in 1.0M HCl on mild steel against concentration of Gnetum africana after 9 hours of exposure at 30ºC |
 | Figure 3. Fourier transform infrared of Gnetum africana.3.4 Temperature considerations |
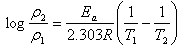 | (3) |
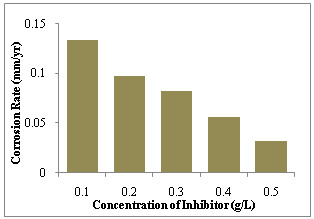 | Figure 4. Corrosion rate of 1.0M HCl on mild steel in the presence of Gnetum africana against concentration of Gnetum africana after 9 hours of exposure at 60ºC |
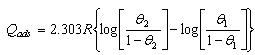 | (4) |
3.4. Adsorption Isotherms
- Assuming a direct relationship between inhibition efficiency and the degree of surface coverage (θ) for different inhibitor concentrations, data obtained from gravimetric measurements were adapted to determine the fit to some well-known adsorption isotherms including the Langmuir, Temkin, Freundlich and the Flory-Huggins isotherms as well as the kinetic-thermodynamic model of El-Awady et al. The generalised expression for the common adsorption isotherms is of the form[33-34]:
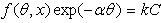 | (5) |
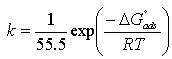 | (6) |
 | Figure 5. Langmuir isotherm for Gnetum africana adsorption on carbon steel in 1M HCl at 30ºC |
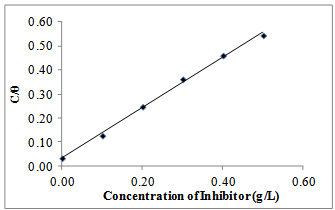
 | Figure 6. Langmuir isotherm for Gnetum africana adsorption on carbon steel in 1M HCl at 60ºC |
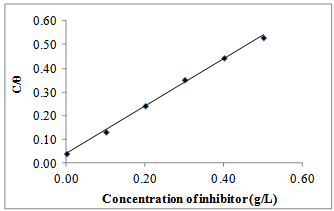
 | Figure 7. Temkin isotherm for Gnetum africana adsorption on carbon steel in 1M HCl at 30ºC |
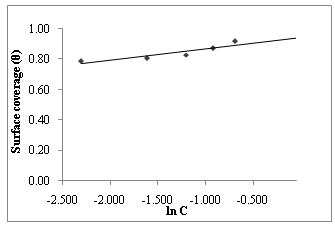
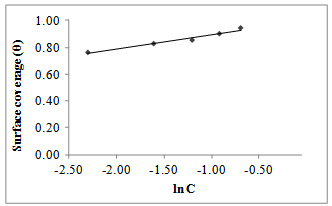 | Figure 8. Temkin isotherm for Gnetum africana adsorption on carbon steel in 1M HCl at 60ºC |
| |||||||||||||||||||||||||||||||||||||||||||||
4. Conclusions
- (1) Gnetum africana acts as a good inhibitor for the corrosion of carbon steel in 1.0 M HCl solution. Inhibition efficiency increases with the inhibitor concentration, and the maximum of 92.42% was obtained at 0.5mg/l concentration.(2) The adsorption of G. africana on C-steel surface obeys the Langmuir adsorption isotherm and is a spontaneous, exothermic process accompanied by an increase in entropy.(3) The corrosion rate of steel in HCl solution without and with G. africana acts as a function of immersion time from 1 to 10 h, with optimum result at the 9th hour of exposure.(4) The FTIR result indicates the presence of a uniform and dense adsorptive film over the steel surface, which efficiently inhibits the corrosion of C-steel.
References
| [1] | G. Trabanelli, "Inhibitors an old remedy for a new challenge", Corrosion, vol.47, pp.410-419, 1991. |
| [2] | A. Popova, E. Sokolova, S. Raicheva, M. Christov, "AC and DC study of temperature effect on mild steel corrosion in acidic media in the presence of benzimidazole derivatives", Corrosion Science, vol.45, no.1, pp.33-58, 2003. |
| [3] | Emeka E. Oguzie, "Corrosion inhibitive effect and adsorption behaviours of Hibiscus sabdariffa on mild steel in acidic media", Portugaliae Electrochemica Acta, vol.26, pp.303-314, 2008. |
| [4] | Emeka E. Oguzie, "Evaluation of some inhibitive effect of some plant extracts on the acid corrosion of mild steel", Corrosion Science, vol.50, pp.2993-2998, 2008. |
| [5] | E. Ebenso, N. Eddy, A. Odiongenyi, "Corrosion inhibition and adsorption properties of methacarbanol on mild steel in acidic medium", Portugaliae Electrochemica Acta, vol.27, no.1, pp.13-22, 2009. |
| [6] | S. B. Ulaeto1, U. J. Ekpe, M. A. Chidiebere, E. E. Oguzie, Corrosion Inhibition of Mild Steel in Hydrochloric Acid by Acid Extracts of Eichhornia Crassipes, International Journal of Materials and Chemistry, vol.2, no.4, pp.158-164, 2012. |
| [7] | P. O. Atanda, O. E. Olorunniwo, O. D. Alabi, O. O. Oluwole., Effect of Iso-Thermal Treatment on the Corrosion Behaviour of Low Carbon Steel (Nigerian C2R grade) in a Buffered Solution containing Chloride and Carbonate Ions, International Journal of Materials and Chemistry, vol.2, no.2, pp.65-71, 2012. |
| [8] | S. Martinez, "Inhibitory mechanism of mimosa tannin using molecular modelling and substitutional adsorption isotherms", Materials Chemistry and Physics, vol.77, pp.97-102, 2002. |
| [9] | E. Ebenso, U. Ekpe, S. Umoren, J. Ekerete, O. Abiola, N. Oforka, S. Martinez, "Corrosion inhibition studies of some plant extracts on aluminium in acidic medium", Journal of Corrosion Science and Technology, vol.1, no.1, pp.96-101, 2004. |
| [10] | O. Abiola, N. Okafor, E. Ebenso, N. Nwinuka, "Eco-Friendly Corrosion Inhibitors: Inhibitive Actions of Delonis regia Extract for the Corrosion of Aluminium in Acidic Medium". Anti-Corrosion Methods and Materials, vol.54, pp.219-224, 2007. |
| [11] | L. Chauhan, G. Gunasekaran, "Corrosion inhibition of mild steel by plant extract in dilute HCl medium", Corrosion Science, vol.49, no.3, pp.1143–1161, 2007. |
| [12] | Mejeha, A. Uroh, K. Okeoma, G. Alozie, "The inhibitive effect of Solanum melongena L. Leaf extract on the corrosion of aluminium in tetraoxosulphate (VI) acid", African Journal of Pure and Applied Chemistry, vol.4, no.8, pp.158-165, 2010. |
| [13] | M. Behpour, S. Ghoreishi, M. Khayatkashani, N. Soltani, "Green approach to corrosion inhibition of mild steel in two acidic solutions by the extract of Punica granatum peel and main constituents". Materials Chemistry and Physics, vol.131, pp.621–633, 2012. |
| [14] | S. Deng, X. Li, "Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions", Corrosion Science, vol.55, pp.407–415, 2012. |
| [15] | U. Eduok, S. Umoren, A. Udoh, "Synergistic inhibition effects between leaves and stem extracts of Sida acuta and iodide ion for mild steel corrosion in 1 M H2SO4 solutions", Arabian Journal of Chemistry, vol.5, no.3, pp.325-337, 2012. |
| [16] | S. Garai, S. Garai, P. Jaisankar, J. Singh, A. Elango, "A comprehensive study on crude methanolic extract of Artemisia pallens (Asteraceae) and its active component as effective corrosion inhibitors of mild steel in acid solution", Corrosion Science, vol.60, pp.193–204, 2012. |
| [17] | H. Gerengi, H. Sahin, "Schinopsis lorentzii extract as a green corrosion inhibitor for low carbon steel in 1 M HCl solution", Journal of Industrial and Engineering Chemistry, In Press, 2012. |
| [18] | L. Li, X. Zhang, J. Lei, J. He, S. Zhang, F. Pan, "Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel". Corrosion Science, vol.63, 82-90, 2012. |
| [19] | X. Li, S. Deng, H. Fu, "Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract", vol.62, pp.163-175, 2012. |
| [20] | N. Soltani, N. Tavakkoli, M. Khayatkashani, M. Jalali, A. Mosavizade, "Green approach to corrosion inhibition of 304 stainless steel in hydrochloric acid solution by the extract of Salvia officinalis leaves", CorrosionScience, vol.62, pp.122-135, 2012. |
| [21] | P. Okafor, M. Ikpi, I. Uwah, E. Ebenso, U. Ekpe, S. Umoren, "Inhibitive action of Phyllanthus amarus on the corrosion of mild steel in acidic medium", Corrosion Science, vol.50, no.8, pp.2310-2317, 2008. |
| [22] | X.H. Li, S.D. Deng, H. Fu, G.N.Mu, "Inhibition effect of 6-benzylaminopurine on the corrosion of cold rolled steel in H2SO4 solution", Corrosion Science, vol.51, pp.620–634, 2009. |
| [23] | Hongda Deng, Chunfu Li, Xianlong Cao, "Effect of carbon dioxide on corrosion of tubular steel in high hydrogen sulfide and carbon dioxide environments", Anti-Corrosion Methods and Materials, vol.58 no.4, pp.196 - 204, 2011. |
| [24] | Q. Qu, S. Jiang, L. Li, W. Bai, J. Zhou, "Corrosion behavior of cold rolled steel in peracetic acid solutions", Corrosion Science, vol.50, pp.35-40, 2008. |
| [25] | Q. Deng, L. Liu, H. Deng, Principles of Spectrometric Identification,2nd Edition, Science Press, Beijing, 2007. |
| [26] | Emeka E. Oguzie, "Corrosion inhibition of aluminium in acidic and alkaline media by Sansevieria trifasciata extract". Corrosion Science, vol.49, pp.1527–1539, 2007. |
| [27] | E. Oguzie, G. Onuoha, E. Ejike, "Effect of Gongronema latifolium extract on aluminium corrosion in acidic and alkaline media", Pigment Resin Technology, vol.36, no.1, pp.44-49, 2007. |
| [28] | Xianghong Li, Shuduan Deng, Hui Fu, "Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract", Corrosion Science, vol.62, pp.163-175, 2012. |
| [29] | Lei Jinglei, Zheng Sha, Li Lingjie, Zhang Shengtao, "Applications of Ellipsometry in Corrosion and Protection of Metals", Corrosion Science and Protetion Technology, vol.24, no.2, pp.91-94, 2012. |
| [30] | A. Yurt, S. Ulutas, H. Dal, "Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some Schiff bases", Applied Surface Science, vol.253, pp.919-925, 2006. |
| [31] | Emeka E. Oguzie, "Influence of halide ions on the inhibitive effect of Congo red dye on the corrosion of mild steel in sulphuric acid solution", Materials Chemistry and Physics, vol.87, pp.212-217, 2004. |
| [32] | K. Kumar, M. Pillai, G. Thusnavis, "Seed Extract of Psidium guajava as Ecofriendly Corrosion Inhibitor for Carbon Steel in Hydrochloric Acid Medium", Journal of Materials Science and Technology, vol.27, no.12, pp.1143, 2011. |
| [33] | H. Ashassi-Sorkhabi, D. Seifzadeh, M.G. Hosseini, "EN, EIS and polarization studies to evaluate the inhibition effect of 3H-phenothiazin-3-one, 7-dimethylamin on mild steel corrosion in 1 M HCl solution", Corrosion Science, vol.50, pp.3363–3370, 2008. |
| [34] | S Martinez. "Inhibitory mechanism of mimosa tannin using molecular modeling and substitutional adsorption isotherms", Materials Chemistry and Physics, vol.77, no.1, pp.97-102, 2003. |
| [35] | S. Shibli, V. Saji, "Co-inhibition characteristics of sodium tungstate with potassium iodate on mild steel corrosion", Corrosion Science, vol.47, no.9, pp.2213-2224, 2005. |
| [36] | A. Ostovari, S. Hoseinieh, M. Peikari, S. Shadizadeh, S. Hashemi, "Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (lawsone, Gallic acid, a-DGlucose and tannic acid) ", Corrosion Science, vol.51, pp.1935–1949, 2009. |
| [37] | A. Satapathy, G. Gunasekaran, S. Sahoo, K. Amit, R. Rodrigues, "Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution", Corrosion Science, vol.51, pp.2848–2856, 2009. |
| [38] | Ruth Villamil, Paola Corio, Silvia Agostinho, Joel Rubim, "Effect of sodium dodecylsulfate on copper corrosion in sulfuric acid media in the absence and presence of benzotriazole", Journal of Electroanalytical Chemistry, vol.472, no.2, pp.112-119, 1999. |
| [39] | Regina Fuchs-Godec, Gregor Žerjav, "Inhibition Properties of Triton-X-100 on Ferritic Stainless Steel in Sulphuric Acid at Increasing Temperature", Acta Chimica Slovenica, vol.56, no.1, pp.78-85, 2009. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML