-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2013; 3(1): 1-4
doi:10.5923/j.ijmc.20130301.01
Synthesis, Crystal Structure and Hydrogen-bonding Patterns in Rac-N-acetyl-2-thiohydantoin-leucine
Gerzon E. Delgado, María E. Sulbaran, Asiloé J. Mora
Laboratorio de Cristalografía, Departamento de Química Facultad de Ciencias, Universidad de Los Andes, Mérida 5101, Venezuela
Correspondence to: Gerzon E. Delgado, Laboratorio de Cristalografía, Departamento de Química Facultad de Ciencias, Universidad de Los Andes, Mérida 5101, Venezuela.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
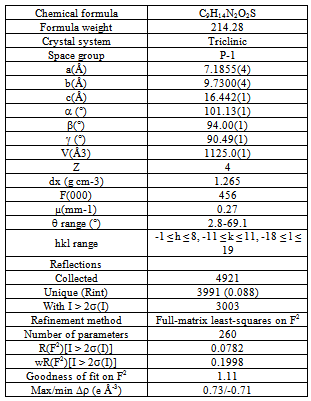
In this work we present the synthesis and X-ray single crystal structural characterization of the heterocyclic compound rac-N-acetyl-2-thiohydantoin-leucine. This material crystallize in the triclinic system with space group P-1 (Nº2), Z=4, with two independent molecules in the unit asymmetric. The crystal packing is governed by N--H···O hydrogen bond-type intermolecular interactions, forming infinite one-dimensional chains with graph-set motif C(6).
Keywords: Thiohydantoin, Crystal Structure, Hydrogen Bonding
Cite this paper: Gerzon E. Delgado, María E. Sulbaran, Asiloé J. Mora, Synthesis, Crystal Structure and Hydrogen-bonding Patterns in Rac-N-acetyl-2-thiohydantoin-leucine, International Journal of Materials and Chemistry, Vol. 3 No. 1, 2013, pp. 1-4. doi: 10.5923/j.ijmc.20130301.01.
Article Outline
1. Introduction
- Thiohydantoins and hydantoins are five-member heterocyclic system with a very reactive nucleus, which provides four possible points of diversity. Both heterocycles represent significant building blocks for combinatorial chemistry libraries[1-4]. The biological activities of hydantoin and 2-thiohydantoin derivatives has been known for a long time, and are responsible for a wide variety of biological behaviour[5], due principally to its wide range of therapeutic properties. For instance, several applications have been reported for hydantoins: antiarrhythmic and antihypertensive[6-7], antiviral[8], antineoplastic[9], antitumoral[10] and anticonvulsant agents[11-12]. The best knwon hydantoin, phenytoin, is the most widely used antiepileptic drug[13]. Thiohydantoins are known for their uses as hypolipidemic[14], antimutagenic[15] and anticarcinogenic agents[16]. In addition, both heterocyclic compounds are used as herbicides[17] and fungicides agents[18]. Recently, there has been interest in the search of new synthetic routes for the preparation of these type of compounds, via solution or solid state reactions[19-21].We are interested in N-carbamoyl, hydantoin and thiohydantoin derivatives of α-amino acids[22-28], and report here the structure of the N-acetylthiohydantoin derivative of the α-amino acid L-leucine.
2. Experimental
2.1. Synthesis
- The title compound was synthesized from L-leucine using a modified methodology previously reported[18-19]. L-leucine (1000 mg, 7.6 mmol) and NH4SCN (580.3 mg, 7.6 mmol) was dissolved in a 9 ml acetic anhydride - 1 ml acetic acid mixture and transferred in a 25 ml round-bottom flask. The mixture was warmed, with agitation, to 363 K over a period of 30 min. The resulting solution was cooled in a ice/water mixture and stored in a freezer overnight. The resulting white solid was filtered off and washed with cool water (m.p. 404-405 K). Crystal of (I) suitable for X-ray diffraction analysis were obtained by slow evaporation of a 1:1 ethanol-methanol solution.RMN-1H (400 MHz, DMSO-d6) δ=12.66 (H3, s), 4.71 (H5, d), 2.70 (H7, s), 1.76 (H8, H9, m), 0.85 (H10, H11, d). RMN-13C (100.6 MHz, DMSO-d6) δ=182.5 (C2), 173.5 (C4), 169.7 (C6), 61.3 (C5), 38.1 (C8), 27.3 (C7), 23.7 (C9), 23.1 (C10), 21.9 (C11).
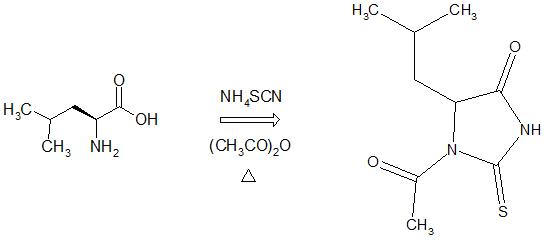 | Scheme 1. Synthesis of rac-N-acetyl-2-thiohydantoin-leucine |
2.2. X-Ray Crystallography
- Colorless rectangular crystal (0.3, 0.3, 0.1 mm) was used for data collection. Diffraction data were collected at 298(2) K by ω-2θ scan technique on a Siemens P4 four-circle diffractometer[29] equipped with graphite monochromatized CuKα radiation (λ = 1.54178 Å). The data were corrected for Lorentz-polarization and absorption effects[29]. Three standard reflections were monitored every 100 reflections (intensity decay: none). The structure was solved by direct methods using the SHELXS97 program[30] and refined by a full-matrix least-squares calculation on F2 using SHELXL97[30].
|
|
|
3. Results and Discussion
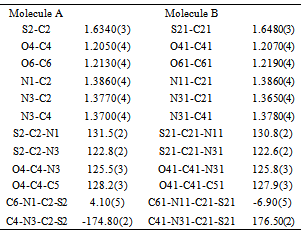
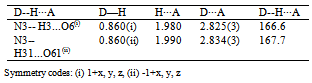
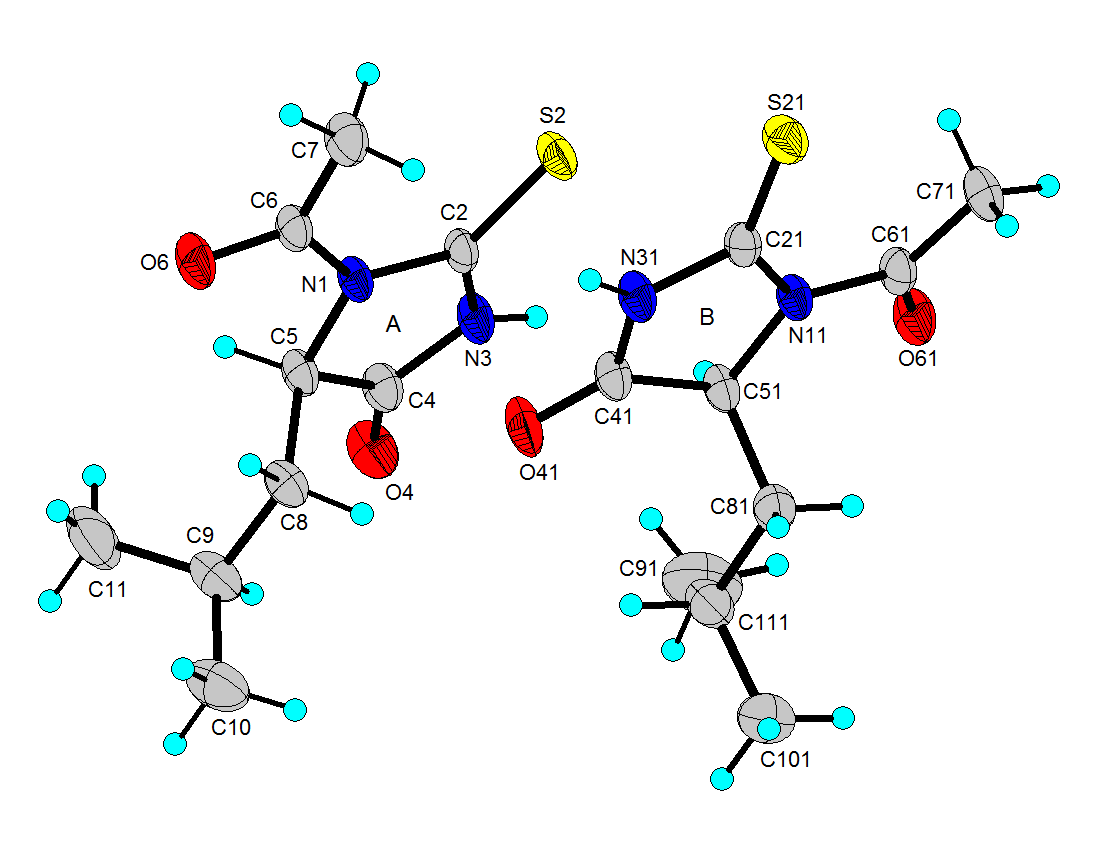
- N-acetyl-thiohydantoin-L-leucine crystallizes with two independent molecules in the asymmetric unit, in a centrosymmetric space group, which implies that L-leucine suffered an amino acid racemization produced by the use of acetic acid in the synthesis[33]. Figure 1 shows the atom labeling and molecular conformation of the two independent molecules of the title compound in the asymmetric unit.All bond distances and angles are normal[34] and are in agreement with the average values found in 31 entries with 36 thiohydantoin ring fragments, searched in the Cam bridge Structural Database (CSD, version 5.33; Feb, 2012) with N1 and N3 unsubstituted and sp3 hybridization at C5. The thiohydantoin ring, in both molecules, is essentially planar with a maximum deviations of 0.034 (3) Å in C4 and −0.037 (3) Å in C4, in molecule A and 0.039(3) Å in C41 and -0.038(3) Å in C51, for molecule B.The S2-C2-N1 bond angles are greater than S2-C2-N3 angles in both molecules. This difference is also observed in the only three 2 N-acetyl thiohydantoin compounds reported in the CSD; KOMGUO[35] with angle values 130.6° and 123.4°, NIFHIT[36] with angles 132.0° and 121.9°, and DIKWAW[28] with angles 132.2°-131.2° and 125.8°-119.0°. The average values for the same angle in the 36 fragments searched above are 127.7° and 125.2°, respectively.The S2-C2 and S21-C21 distance values, see Table 2, agree with the average value of 1.646 Å found for the 36 fragments search in the CSD, with minimal and maximum reported values of 1.519 and 1.696 Å, respectively. The molecular structure and crystal packing of the title compound is stabilized by intermolecular N3--H3···O4 (x, 1/2 − y, 1/2 + z) hydrogen bonds (Table 3), forming infinite one-dimensional chains that run along[100] direction, which can be described in graph-set notation as C(6)[37] (Figure 2).
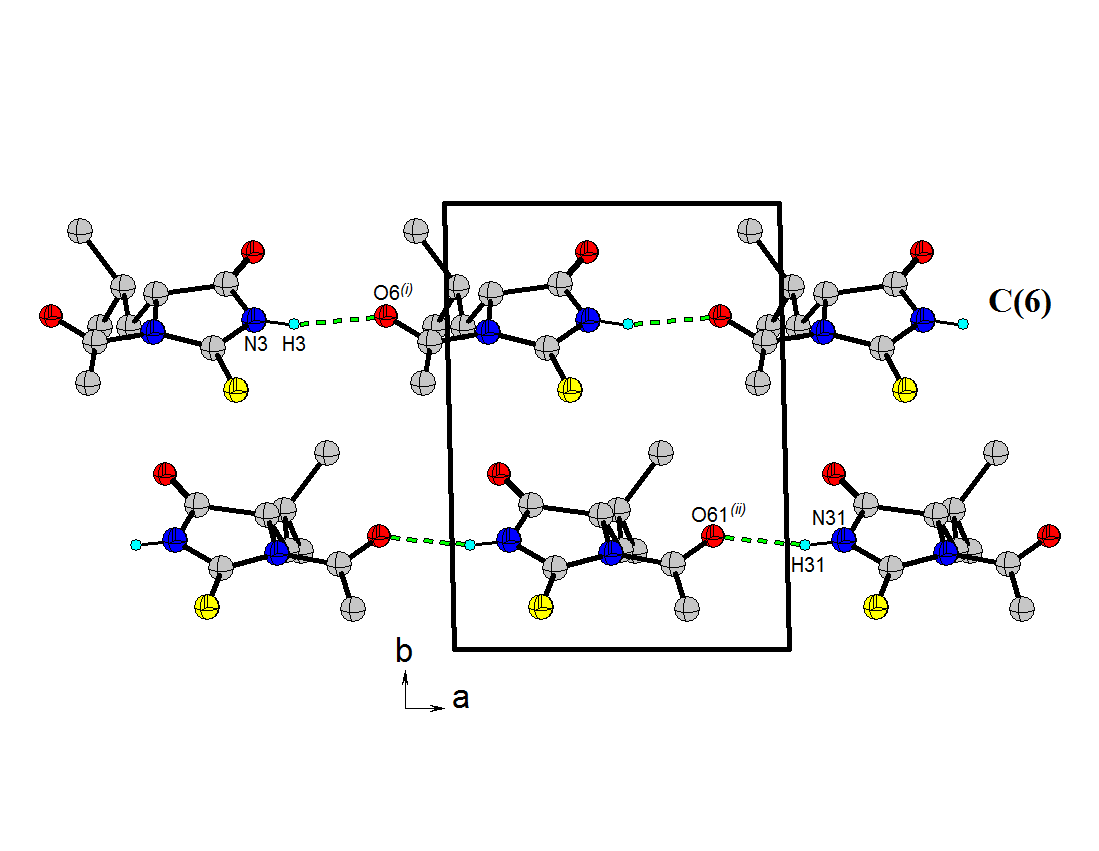 | Figure 2. Packing view of 1. Intermolecular hydrogen bonds, N--H···O and O--H···O, are indicated by dashed lines. H atoms not involved in hydrogen bonding have been omitted for clarity |
4. Conclusions
- The thiohydantoin derivative rac-N-acetyl- 2-thiohydantoin-leucine was synthesized and characterized by NMR spectroscopy. This material crystallize in the triclinic system with space group P-1 (Nº2), Z=4, with two independent molecules in the unit asymmetric. In the crystal structure, the molecules are linked by N---H···O hydrogen bonds, forming infinite one-dimensional zigzag chains, running along [100] direction, with C(6) graph-set motif.
ACKNOWLEDGEMENTS
- This work was supported by CDCHT-ULA (grant C-1616-08-08-A) and FONACIT (grant LAB-97000821). The authors thank H.N. de Armas and N. Blaton, Katholieke Universiteit Leuven, Belgium, for data collection.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML