-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2012; 2(5): 218-224
doi: 10.5923/j.ijmc.20120205.06
Controlled Preparation and Surface Structure Characterization of Carbon-Coated Lithium Iron Phosphate and Electrochemical Studies as Cathode Materials for Lithium Ion Battery
Xiangcheng Sun1, Kai Sun2, Caiyun Chen3, Haiping Sun2, Bo Cui1
1Department of Electrical and Computer Engineering, University of Waterloo, Waterloo, Canada
2Department of Materials Science and Engineering, University of Michigan, Ann Arbor, Michigan 48109 US
3Department of Materials Science and Engineering, Shandong University of Technology, Zibo, PR China
Correspondence to: Xiangcheng Sun, Department of Electrical and Computer Engineering, University of Waterloo, Waterloo, Canada.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Amorphous carbon-coated lithium iron phosphate (C-LiFePO4) particles have been mass synthesized at the commercial scale by a controlled solid-state reaction method. Particles morphologies, olivine-type phase structures and the carbon coating features were investigated in details by various techniques as X-ray diffraction analysis (XRD), scanning electron microscopy (SEM) imaging, and transmission electron microscopy (TEM, HRTEM) imaging, selected-area electron diffraction (SAED), X-ray energy dispersive spectroscopy microanalysis (XEDS), and X-ray photoelectron spectroscopy (XPS). Single-crystal nature of the olivine type LiFePO4 structures was revealed by XRD and SAED analyses. TEM imaging showed rough nanoparticles spherical features with average size range of 50-200 nm. Homogenous coating features of carbon layers on the particles surface and olivine-LiFePO4 phase are clearly observed in HR-TEM imaging and confirmed by the corresponding SAED pattern. Elemental binding energy from XPS analysis also confirmed that an amorphous sp2 carbon coating layer and olivine type LiFePO4 structures. It is indicated that the characteristics of sp2 type conducting-coating layer on the particles surfaces gave rise to improved electrical conductivity by reducing the diffusion path of the electron and lithium ions, as directly evidenced from our charge-discharge cycling testing as the cathode in the Lithium ion battery cell.
Keywords: Lithium Iron Phosphate, Olivine-Type Phase, X-Ray Diffraction, Transmission Electron Microscopy, X-Ray Photoelectron Spectroscopy, Electrochemical Capacity
Cite this paper: Xiangcheng Sun, Kai Sun, Caiyun Chen, Haiping Sun, Bo Cui, "Controlled Preparation and Surface Structure Characterization of Carbon-Coated Lithium Iron Phosphate and Electrochemical Studies as Cathode Materials for Lithium Ion Battery", International Journal of Materials and Chemistry, Vol. 2 No. 5, 2012, pp. 218-224. doi: 10.5923/j.ijmc.20120205.06.
Article Outline
1. Introduction
- Extensive attention has been devoted to the development and characterizations of the phospho-olivine type lithium iron phosphate (LiFePO4) as an attractive cathode candidate in the lithium ion battery market, which was first reported by John Goodenough in 1997 as a promising cathode electrode for the rechargeable lithium-ion batteries[1]. Olivine-type LiFePO4 exhibits various unique advantages such as low toxicity, low cost, high thermal and chemical stability, and good electrochemical performance in the fully charged state, and it also has a higher theoretical specific capacity (170 mAh g−1) and a flat charge–discharge profile at intermediate voltage (3.45 V vs Li/Li+), and reasonable cycle life[2-3]. However, LiFePO4 has inherently low electrical conductivity, which results in its poor rate capability due to the poor kinetics of lithium intercalation/deintercalation process [2-15]. This causes a bigger challenge for power-demanding applications such as hybrid electric vehicles and electric vehicles[2-6]. Nowadays many approaches have been successfully adopted to improve its electrical conductivity, such as metal cation doping and carbon-coating or addition[7-12], decreasing the particle size and producing nanoparticles[13-18]. It has been reported thatcarbon-coating would increase the surface electrical conductivity and smaller size of LiFePO4 could shorten the diffusion length of Li-ion during lithiumintercalation/deintercalation process[3, 6, 20]. Particularly, it is apparent that carbon coating has been reported to be very effective in the enhancement of capacity and rate capability on LiFePO4 cathode[6-20, 24-33]. In the present work, we report large-scale commercial synthesis and structural morphologies of carbon coated lithium iron phosphate (C-LiFePO4) particles prepared using home-made amorphous micro-FePO4 as the iron source and conducting black as carbon source. Furthermore, we examined the electrochemical performance of the C-LiFePO4 particles as a cathode for the lithium-ion battery.
2. Experiments
2.1. Sample Preparation
- First of all, in order to produce the homogenous carbon-coated LiFePO4 products, the choice of suitable chemical precursors and starting reagents is very essential. Meanwhile, the appropriate temperature and pressure must be carefully selected to prevent the undesirable particle growth and the presence of impurity phase. Otherwise, undesired impurities, such as Fe2O3 and Fe2P, can be contained in the final products. Here, micro-FePO4 powders were firstly synthesized via the solid-state reaction (i.e. pre-calcination), which was typically conducted by heating the mixture of the relatively cheap precursors of FeSO4 and NH4H2PO4 (e.g. FeSO4:NH4H2PO4=1:2) at up to 300℃. Consequently, the appropriate stoichiometric amount (e.g. 2:1) of amorphous micro-FePO4 powders and Li2CO3 powders were deeply calcinated under the high-temperature carbonization with presence of the conductive carbon source (i.e. acetylene black) in an industrial-type furnace. All the reagents used in the experiment are of analytical purity. Usually, the high temperature solid state calcination was systemically carried out under controlled temperature ranges (700℃-800℃) and certain pressure for over 5-10 h. By controlling the calcination temperature and atmosphere pressures, the LiFePO4 powders products could be produced maximum at 5kg/day. The yield purity of the as-prepared LiFePO4 powders was up to 95% that collected in the bag filter regardless of the conducting carbon sources.
2.2. Sample Characterization
- The phase purity and microstructure were characterized and recorded by XRD using a D/max−2000 Rigaku diffractometry with Cu Kα radiation (λ = 0.15406 nm) operated at 40 kV and 30 mA within the scanning angle (2θ) ranging from 20° to 70°, and a step of 0.01°.The morphologies of as-prepared particles were examined using a scanning electron microscopy (Carl Zeiss, InLens, WD = 9.6, 15Kv). The dispersed particles supported on a 200 mesh holy carbon film coated Cu grids were characterized at nanoscale by a conventional transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HR-TEM), and selected area electron diffraction (SAED) techniques using a JEOL 3010 microscopy equipped with XEDX system at 300 kV. Surface chemical elements characterizations of particles were determined and recorded by X-ray photoelectron spectrometer (XPS) using a Kratos ULTRA DLD XPS with a mono-chromated Al source that gives an energy resolution better than 0.5 eV at a pass energy smaller than 20eV. Both survey scans (with a pass energy of 160 eV and a scan step of 1eV) and core scans (with a pass energy of 20 eV and a scan step of 0.1eV) were collected. The spectra were calibrated by setting the C 1s peak as 285 eV. Electrochemical properties were measured on electrodes prepared by using the mixtures of comprising 85 wt% active materials, 10 wt% acetylene black, and 5 wt% polyvinylidene fluorides (PVDF) binder. The LiFePO4 electrode films were fabricated by doctor blade technique on aluminum foil. The cells consisted of the electrode, a lithium metal counter electrode and the electrolyte of a 1 M solution of LiPF6 in ethylene carbonate/dimethyl carbonate (EC/DMC, 1:1). The cells were assembled and handled in an Ar-filled glove box and were evaluated using coin-type cells. Galvanostatic discharge/charge tests were carried out using a program-controlled LAND Celltest-2001A system between 2.5 V and 4.2 V versus the Lithium counter electrode at room temperature.
3. Results and Discussion
- The powder X-ray diffraction pattern (XRD) taken from the as-prepared particles is shown in Fig.1. It clearly revealed that the C-LiFePO4 particles were indexed well to a pure orthorhombic system of olivine-type structure (Pnma, JCPDS: 40-1499). No any impurities phase such as transition metal compounds Li3PO4 and FePO4 were detected by XRD. Carbon phase was not found apparently from the XRD pattern, which indicates it may exist in amorphous form.
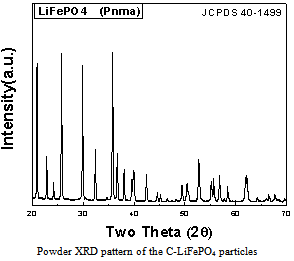 | Figure 1. Powder XRD pattern |
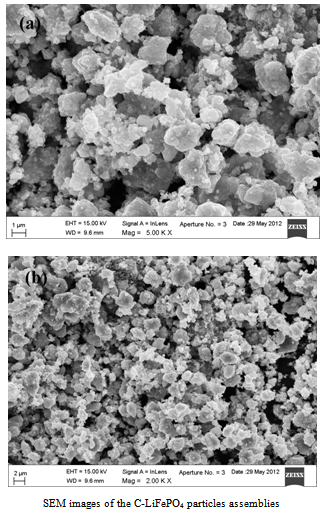 | Figure 2(a,b). SEM images |
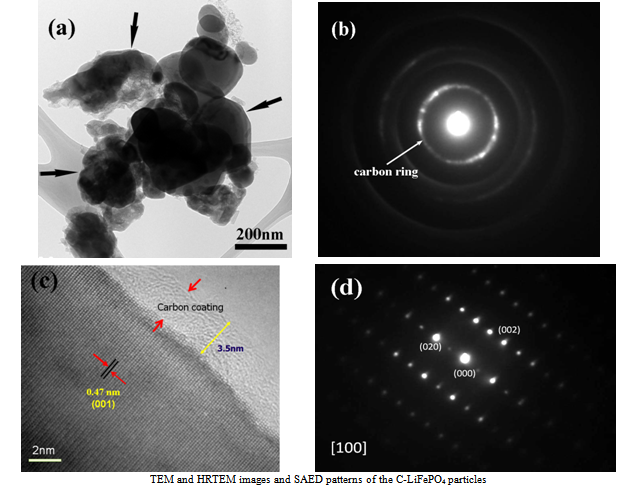 | Figure 3(a,b,c,d). TEM and HRTEM images and SAED pattern |
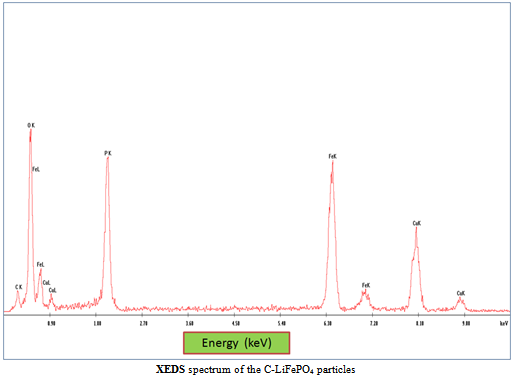 | Figure 4. The XEDS spectrum |
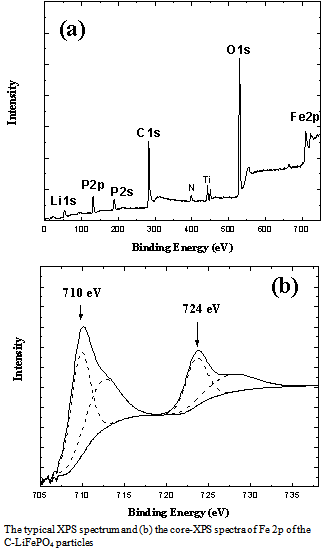 | Figure 5 (a) The typical XPS survey spectrum and (b) the core-scan spectra of Fe 2p |
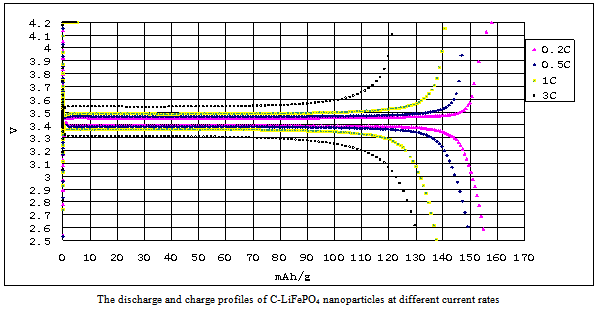 | Figure 6. The discharge and charge profiles |
4. Conclusions
- 1), Carbon coated LiFePO4 (C-LiFePO4) powders products with the olivine-type structure has been successfully synthesized at commercial scale. XRD, SEM, SAED, HR-TEM image, and XPS analyses clearly provide a comprehensive view of the structure-correlated performance of the C-LiFePO4 products. It demonstrates that each LiFePO4 particle has a sp2 amorphous carbon-coating shell and a phospho-olivine type crystal core. 2), The unique olivine-type C-LiFePO4, combined with its full coating of conductive carbon, effectively enhances its electrochemical performance due to the presence of the carbon coating networks for both electronic and ionic transport increasing during the electrochemical testing. 3), The present mass production procedure is very valuable to optimize the process for producing carbon coated LiFePO4 cathode materials, this method is likely to be easy to scale up for industrial production.
ACKNOWLEDGMENTS
- This works are financially support from the President’s Award of University of Waterloo and Postgraduate Scholarship of the Natural Sciences and Engineering Research Council of Canada (NSERC). Thanks to Jiangsu Fangzhou New Energy Company, China for offering me the collaborations for the samples preparations. Thanks also give to Prof. Jinbo Yang and Mr. Xuegang Chen for their kind XRD analysis and SEM measurements at School of Physics, Beijing University, China.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML