-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2012; 2(5): 192-196
doi: 10.5923/j.ijmc.20120205.02
Vilsmeier-Haack Reaction of Phosphonic Dihydrazide: Synthesis of 4- {[(Dimethyl)azanylidenonium Chloride] Methyl} Amino-2,3-Dihydro-3-Oxo-4H-1,2,4, 3-Triazaphosphole
Tarik E. Ali, Somaia M. Abdel-Kariem
Synthetic organic laboratory, Department of Chemistry, Faculty of Education, Ain Shams University, Roxy, 11711, Cairo, Egypt
Correspondence to: Tarik E. Ali, Synthetic organic laboratory, Department of Chemistry, Faculty of Education, Ain Shams University, Roxy, 11711, Cairo, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A simple method for the synthesis of 4-{[(dimethyl)azanylidenonium chloride] methyl}amino-2,3-dihydro- 3-oxo-4H-1,2,4,3-triazaphosphole (2) is described. Treatment of phosphonic dihydrazide (1) with Vilsmeier-Haack reagent led directly to the title compound in high yield.
Keywords: Phosphonic Dihydrazide,Vilsmeier Reagent, 1,2,4,3-Triazaphosphole
Cite this paper: Tarik E. Ali, Somaia M. Abdel-Kariem, "Vilsmeier-Haack Reaction of Phosphonic Dihydrazide: Synthesis of 4- {[(Dimethyl)azanylidenonium Chloride] Methyl} Amino-2,3-Dihydro-3-Oxo-4H-1,2,4, 3-Triazaphosphole", International Journal of Materials and Chemistry, Vol. 2 No. 5, 2012, pp. 192-196. doi: 10.5923/j.ijmc.20120205.02.
Article Outline
1. Introduction
- A Literature search of the last decade revealed sustained interest in the application of the Vilsmeier Haack reagent in organic synthesis. The versatility of the reagent had been extended to activating agents as oxygen and nitrogen nucleophiles to yield the corresponding iminium salts[1-5]. On the other hand, phosphorus heterocycles containing nitrogen heteroatoms adjacent to phosphorus atom exhibited interesting biological properties[6,7]. As a part of our search for new expected biologically active phosphorus heterocycles[8-11], we are interested in introducing such N−P−N pattern into heterocyclic structure by applying the Vilsmeier Haack reaction on phosphonic dihydrazide. To the best of our knowledge, the synthesis of phosphorus heterocycles by the Vilsmeier reagent has not been reported in literature.
2. Experimental
2.1. General
- The melting point was determined in an open capillary tube on a digital Stuart SMP-3 apparatus. Infrared spectrum was measured on Perkin-Elmer 293 spectrophotometer (cm-1), using KBr disks. 1H NMR spectrum was measured on Gemini-200 spectrometer (200 MHz), using DMSO-d6 as a solvent and TMS (δ) as the internal standard. 13C and 31P NMR spectra were registered on a Varian Inova 500 MHz spectrometer at room temperature using DMSO-d6 as a solvent and TMS as internal standard and 85% H3PO4 as external reference. Elemental microanalysis was performed at microanalysis center in National Research Center, Giza. The purity of the synthesized compound was checked by thin layer chromatography (TLC). Phosphonic dihydrazide was prepared according to the reported method[12].
2.2. Synthesis of {[(dimethyl)azanylidenonium chloride]methyl}amino-2,3-dihydro-3-oxo-4H-1,2,4,3-triazaphosphole (2)
- The Vilsmeier reagent was prepared by adding dimethylformamide (3.86 mL, 50 mmol) in an ice-cold condition (0-5℃) under constant stirring. To this, phosphorus oxychloride (1.304 mL, 14 mmol) was added dropwise over a period of 30 minutes and the resulting mixture was stirred for a further 30 minutes. Phosphonic dihydrazide (1) (550 mg, 5 mmol) was added to the Vilsmeier reagent and stirred for 4 hours at 50-60℃. The reaction mixture was cooled and poured crushed ice (30 g) under constant manual stirring. The reaction mixture was kept aside overnight. After adding acetone, the precipitate obtained was washed well with acetone. The product was filtered off and crystallized from acetone/water affording white crystals in 86% yield, m.p. 230−232 ℃. IR (νmax, cm-1): 3474−3150 (h-bonded, OH and NH), 2778 (P−H), 1713 (C=N+), 1661 (C=N), 1291 (P=O). 1H NMR (DMSO): δ 3.01 (s, 6H, 2 CH3), 6.00 (br, 1H, NH exchangeable with D2O), 8.35 (d, 1H, JPH=328 Hz, P−H), 8.05, 8.17 (dd, 1H, J=14 Hz, CH=Nexocyclic), 8.40 (s, 1H, CH=Nendocyclic), 11.32 (br, 1H, NH exchangeable with D2O). 13C NMR (DMSO): 45.2 (CH3), 47.3 (CH3), 158.1 (C=Nexocyclic), 166.3 (C=Nendocyclic). 31P NMR (DMSO): δ 7.58 ppm. MS (I %): 226 (M+1, 7%), 225 (M+, 8%), 99 (3), 98 (17), 71 (42), 70 (24), 57 (100). Anal. Calcd for C4H11ClN5OP. 3/4H2O (225.10): C, 21.32; H, 5.55; N, 31.09. Found: C, 21.43; H, 5.62; N, 30.74%.
3. Results and discussion
- The Vilsmeier-Haack reaction[13] was applied to phosphonic dihydrazide (1) to give 4-{[(dimethyl) azanylidenonium chloride]methyl}amino-2,3-dihydro- 3-oxo-4H-1,2,4,3-triazaphosphole (2). A possible mechanism for this reaction could involve attack of the chloromethyleniminium species obtained in situ from phosphorus oxychloride and dimethylformamide to react with the terminal amino groups of 1 leading to the iminium intermediate A. The intramolecular cyclization of A through addition of NH group adjacent to phosphorus atom to C=N+ group afforded the cyclic intermediate B, which underwent removal of one molecule of dimethylamine to give the isolated product 2. Unfortunately, our attempts to hydrolyze of 2 were unsuccessful under different basic condition which resulted in the formation of a tarry material instead of the expected aldehyde 3, and we failed to isolate individual compound[14]. elemental analysis and mass spectrometry indicated a formula with composition C4H11ClN5OP. 3/4H2O (225.10). This is explained by the presence of a water in the crystallized form, i.e. the molecular formula of the proposed structure is the hydrated form of C4H11ClN5OP. Its IR spectrum displayed OH, NH, C=N+ and C=N functions at 3474−3150, 1713 and 1661 cm-1, respectively[15] (Figure 1). Also, its 1H NMR spectrum exhibited two characteristic signals at δ 8.35 and δ 8.40 ppm corresponding to P−H and CH=Nendocyclic, respectively. Moreover, the proton of CH=N+exocyclic appeared as doublet doublet at δ 8.05 and 8.17 ppm with coupling constant around 14 Hz[16], along with the signals of NH protons which appeared at δ 6.00 and 11.32 ppm which are replaceable with deuterium on addition of D2O (Figure 2). Furthermore, its 13C NMR spectrum supported the suggested structure which showed the methyl carbon atoms at δ 45.2 and 47.3 ppm, while the exocyclic and endocyclic C=N atoms appeared at δ 158.1 and 166.3 ppm, respectively (Figure 3). Finally, 31P NMR spectrum displayed a signal at δ 7.58 ppm.
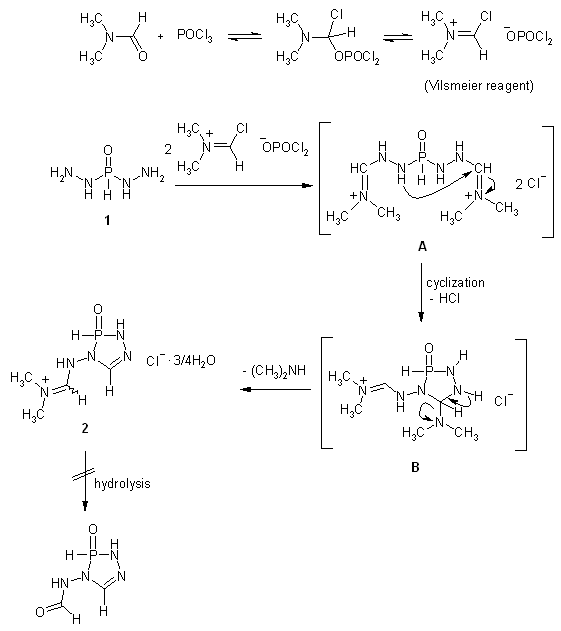 | Scheme 1. The synthetic pathway and its mechanism for formation 2 via applying Vilsmeier-Haack reaction on phosphonic dihydrazide 1 |
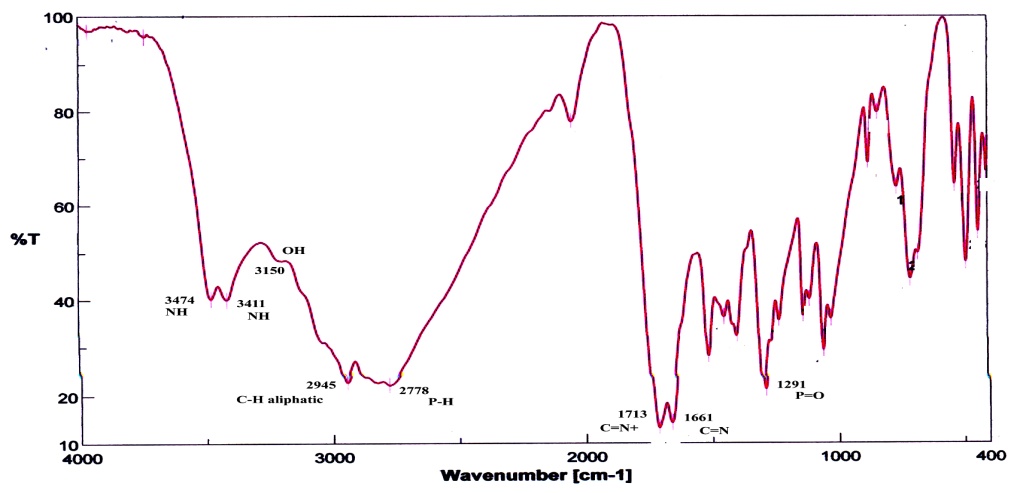 | Figure 1. The IR spectrum of compound 2 |
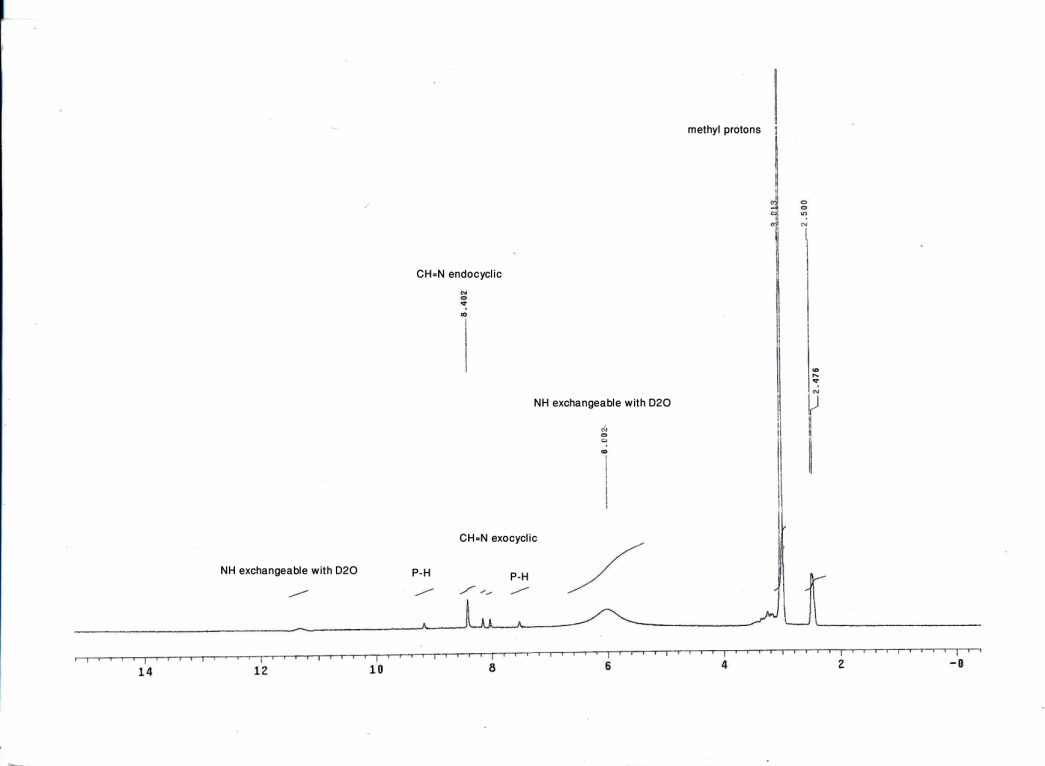 | Figure 2. The 1H-NMR spectrum of compound 2 |
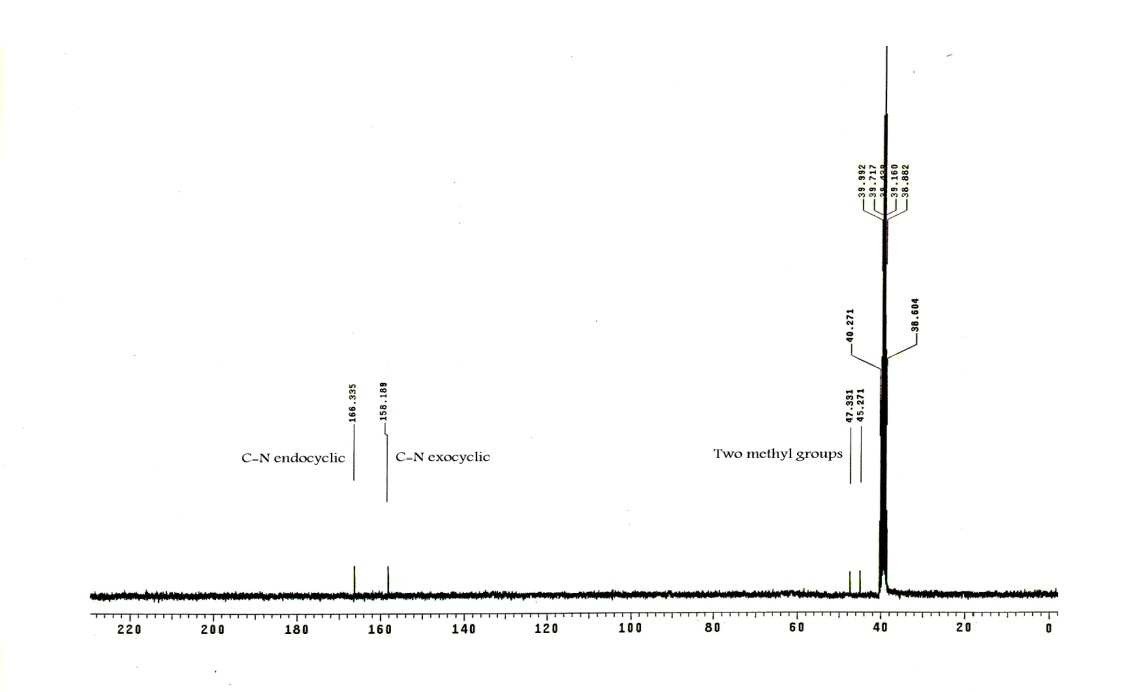 | Figure 3. The 13C-NMR spectrum of compound 2 |
4. Conclusions
- 4-{[(Dimethyl)azanylidenoniumchloride]methyl}amino-2,3-dihydro-3-oxo-4H-1,2,4,3-triazaphosphole (2) is achieved in high yield via treatment of phosphonic dihydrazide (1) with Vilsmeier-Haack reagent.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML