-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2012; 2(4): 178-184
doi: 10.5923/j.ijmc.20120204.11
Effects of Heat Treatment on the Electrochemical Corrosion Behaviour of Aluminum Alloy AA8011 in 0.1M H2SO4 Aqueous Acid Media
Okeoma Kelechukwu B. 1, Owate Israel O. 2, Oguzie Emeka E. 3, Mejeha Ihebrodike M. 1
1Materials Science Group, Department of Physics, Federal University of Technology, Owerri, Nigeria
2Materials Science Group, Department of Physics, University of Port Harcourt, Nigeria
3Electrochemistry and Materials Science Research Unit, Department of Chemistry, Federal University of Technology, Owerri, Nigeria
Correspondence to: Okeoma Kelechukwu B. , Materials Science Group, Department of Physics, Federal University of Technology, Owerri, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The effects of heat treatment and quenching regimens on the electrochemical corrosion behaviour of aluminium alloy AA8011 in 0.1M H2SO4 was studied by open circuit potential (OCP), potentiodynamic polarization (PP) and electrochemical impedance spectroscopy (EIS) measurements. Three different specimens (unheated, air quenched and oven quenched) were investigated. Polarization results show that all the specimens underwent active dissolution, with no distinct transition to passivation. Heat treatment caused the corrosion potential to shift; the oven quenched shifted to the more cathodic region, while the air quenched shifted to the anodic values. There was decrement in the rate of both cathodic and anodic partial reactions of the corrosion processes in the heat treated samples. The impedance spectra for all the specimens comprised of a high frequency capacitive loop and an inductive loop at low frequency and depict higher values of the charge transfer resistance for the heat treated specimens. All the results indicate that heat treatment increased the corrosion resistance of AA8011 in 0.1M H2SO4 with modifying the corrosion mechanism. The corrosion resistance obtained from the impedance measurements increased in the order unheated< air quenched < oven quenched. This trend has been correlated with the phase constituents of the different specimens as determined from the X- ray diffraction (XRD), and scanning electron microscopy (SEM).
Keywords: AA8011, Heat treatment, Corrosion, Polarization, Impedance Spectroscopy, XRD, SEM
Cite this paper: Okeoma Kelechukwu B. , Owate Israel O. , Oguzie Emeka E. , Mejeha Ihebrodike M. , "Effects of Heat Treatment on the Electrochemical Corrosion Behaviour of Aluminum Alloy AA8011 in 0.1M H2SO4 Aqueous Acid Media", International Journal of Materials and Chemistry, Vol. 2 No. 4, 2012, pp. 178-184. doi: 10.5923/j.ijmc.20120204.11.
Article Outline
1. Introduction
- Low density, good thermal conductivity, satisfactory mechanical and relatively good corrosion resistance are among the properties exploited in the usage of aluminium and its alloys in widespread industrial and domestic applications[1-3]. Particularly, aluminium alloy AA8011 is widely used in thin foil production, containers, food packaging, household foils[4, 5]. In extruded form, the alloy system is used in building, frames, automotives and aerospace industry[6]. Due to rise in demand andapplications, research into the usage and the properties of this alloy when exposed to various environments has become of interest. This leads to the harnessing of special qualities of AA8011 offered as result of the elements which this alloy is composed of, coupled with the special characteristics exhibited on heat treatment and during production. For example, silicon which is present in this alloy in quantity nearly as large as iron limits the super-saturation of the matrix phase with Fe by causing a substantial level of Fe precipitation Deligic et al.[5] and Ney and Luiggi[7] studied the role of Si and Fe content on strain hardening and concluded that Fe controls the precipitation kinetics of the material. The micro-structural changes during annealing as studied by Oscarson et al.[8] showed a transition from discontinuous to continuous recrystallization as deformation increased. While Anderson et al.[9] looked at the influence of microstructure and texture on the earing behavior of Al-Fe-Si alloy system. The microstructural control in aluminium core alloy for brazing sheet application showed that an Al-Mn alloy X800 developed significantly increase in the corrosion resistance of radiator tube when exposed to service environment[10]. This behavior can be attributed to the precipitations and crystallization effects as observed by Home[11]. Wang and Jiao[4] equally observed microsegregation of a variant Al-Fe-Mn-Si system and noted the need to reduce Mn in solid solution so as to obtained desirable size of fine dispersiods. This reduction follows the transformation of the intermetallic particle[12]:
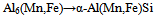 | (1) |
2. Experimental
2.1. Materials Preparation
- Aluminium alloy AA8011 used in this study was obtained from First Aluminum PLC Port Harcourt, Nigeria and had the following weight-percentage composition (Si- 0.24; Fe -0.98; Cu - 0.035; Mn - 0.10; Mg = 0.009; Zn- 0.044; Ti -0.019; Al- 98.52; others the balance) The heat treated specimens were subjected to a temperature of 150℃ in a furnace for 1 h, and subsequently divided into two sets; one set was removed from the furnace and allowed to cool in open air, while the other set was left in the furnace to cool at a rate of 0.2℃/h in order to mimic the gradual cooling sequence found in heat exchanger chambers. The untreated, air-quenched and oven-quenched samples were all machined into coupons of dimensions 1.5 cm x 1.5 cm, which were wet-polished with silicon carbide abrasive paper (from grade #200 to #1000), degreased in acetone, rinsed with distilled water and dried in warm air. These were subsequently sealed with epoxy resin in such a way that only one square surface of area 1.0 cm2 was left uncovered.
2.2. Materials Characterization
- Structural characteristics of the as received AA8011 and heat treated and quenched specimens were analysed by X-ray diffraction (XRD, XPERT-PRO) using Cu Kα radiation as well as by scanning electron microscopy (SEM, Shimadzu SSX-550). XRD measurements were undertaken to enable verification of the phase constitution before and after heat treatment/quenching. SEM imaging was used to identify surface defects (including inhomogeneities and porous intensity) arising from the different heat treatment/quenching regimen.
2.3. Electrochemical Measurements
- Electrochemical experiments were conducted in a conventional three-electrode cell using a VERSASTAT 400 Complete DC Voltammetry and Corrosion System, with V3 Studio software. A platinum foil was used as counter electrode and a saturated calomel electrode (SCE) as reference electrode. The later was connected via a Luggin’s capillary. The test electrolyte was 0.1 M H2SO4, prepared from analytical reagent grade concentrated acid using distilled water. Measurements were performed in an aerated and unstirred solutions at the end of 1 h of immersion at 30℃. Impedance measurements were made at corrosion potentials (Ecorr) over a frequency range of 100 kHz – 10 mHz, with a signal amplitude perturbation of 5 mV. Potentiodynamic polarization studies were carried out in the potential range -0.85 to -0.45V versus the corrosion potential at a scan rate of 1mVs-1. Each test was run in triplicate to verify the reproducibility of the systems.
 | Figure 1. The XRD spectra of unheated, air quenched and oven quenched samples of AA8011 |
3. Results and Discussion
3.1. Structural Analysis
3.1.1. XRD spectra
- Fig.1 shows the XRD patterns of the control, air quenched and oven quenched specimens of AA8011. It is easily seen that all the samples display distinct, sharp and narrow peaks signifying high crystalline nature of AA8011 alloy. The crystallite size was determined using Debye- Scherrer’s formula:[15]
 | (2) |
3.1.2. SEM Morphological Images
- SEM micrographs of unheated, air quenched, and oven quenched specimens are illustrated in Fig 2. The unheated specimen (Fig2a) has many large, white strands, with some clustered dark spots. Fig 2b shows the oven quenched specimen, in which scanty white spots are found. In Fig.2c is the micrograph specimen for air quenched sample. It hardly contains any white spots, but some large circular dark patches are evident. From these specimens, it is clear that heat treatment dissolved the large white spots which are found in the unheated sample.
 | Figure 2. XRD spectra of unheated (a), Oven quenched (b) and air quenched (c) samples of AA8011 |
3.2. Electrochemical Spectroscopy
3.2.1. Open Circuit Potential Measurements
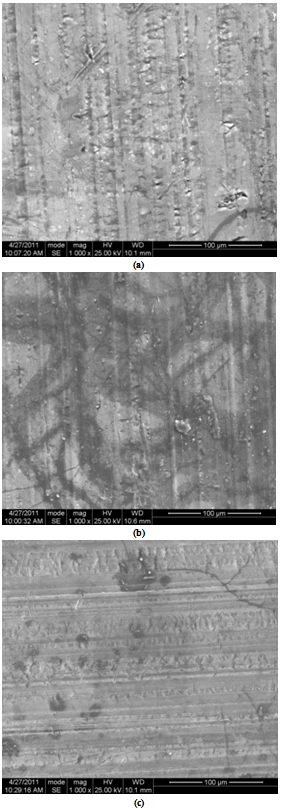
- The evolution of the open circuit potential (Eocp) with time for AA8011 specimens in 0.1M H2SO4 solution is shown in Fig.3. A steady decrease in Eocp is observed for the control specimen due to the dissolution of air formed oxide film on the electrode surface. Both heat treated samples had their Eocp shifted into more positive potentials. This could be attributed to the dissolution of the precipitated intermetallic particles on the alloy surface after heating. The Eocp on the air quenched specimen remained almost constant in the first 2750s before slightly decreased at the end of 1 hour. But for oven quenched specimen, the Eocp decreased almost linearly within the time intervals of 250 and 2750s. It can be concluded that the heat treatment caused the oxide film to decrease the insulating properties, since high insulating films is associated with more cathodic potential as seen in the control sample.[18].
 | Figure 3. Open circuit potential versus time for control, oven quenched and air quenched sample of AA8011 in 0.1 M H2SO4 acid medium |
3.2.2. Potentidynamic Polarization Measurements
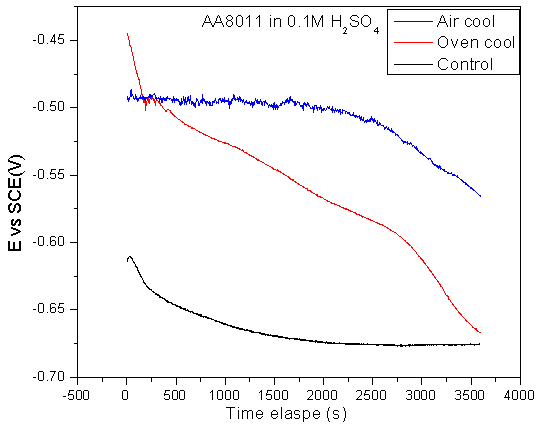
- The potentiodynamic polarization curves for control, oven quenched and air quenched specimens of AA8011 in 0.1 M H2SO4 are presented in Fig.4 and the corresponding electrochemical parameters given in Table 1. All the three samples exhibit active dissolution with no distinctive transition to passivation up to -0.45V versus SCE. The similarity of the polarization curves for the three specimens suggest comparable corrosion mechanism. It is obvious that heat treatment has a positive influence on the polarization behaviour of AA8011 in 0.1M H2SO4 acid environment. It decreased both anodic and cathodic current densities and shifted Ecorr of the oven quenched specimen into more negative potentials. While the Ecorr of the air quenched sample shifted to higher potential. The cathodic arm of the oven quenched and anodic arm of the air quenched specimen shifted to the lower current densities when compare with the control. This translated to increase in polarization resistance and hence decrease in the corrosion current densities. The positive effects of heat treatment may be attributed to the formation of less harmful intermetallic crystallites Al6(Fe,Mn) for air quenched and FeMn for oven quenched samples, whose size and frequency increased as obtained from the XRD data.[19]
 | Figure 4. Potentiodynamic polarization curves for control, oven quenched and air quenched samples AA8011 in 0.1M H2SO4 acid medium |
3.2.3. Impedance Measurements
- Electrochemical impedance spectra were measured at the open circuit potential for the different AA8011specimens in 0.1M H2SO4 solution. The obtained impedance responses are presented in Nyquist of Fig.5. All the specimens display similar impendence features; each Nyquist plot is characterized by a single depressed capacitive semicircles from high to intermediate frequencies followed by a low frequency inductive arc. The capacitive loop can be attributed to charge transfer processes associated with the effects of the double layer capacitance and its diameter is related to the charge transfer resistance (Rct) at the metal/solution interface, while the inductive loop probably results from the intermediate products. The Nyquist plots clearly indicate that heat treatment caused increase in the diameter of the capacitive arc, hence the Rct which corresponds to an increase in the corrosion resistance of AA8011 in 0.1 M H2SO4 solution. This effect is more pronounced for oven quenched sample and points toward the low corrosion susceptibilities of the heat treated samples in the sulphuric acid medium. Rct increase in the order unheated< air quenched < oven quenched. This does not agree with the trend of corrosion resistance predicted by the polarization data, possibly because of effects of different heat treatment on the Tafel constant.
 | Figure 5. Electrochemical impedance spectra of unheated/ control, oven quenched and air quenched specimens of AA8011 in 0.1M H2SO4 |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
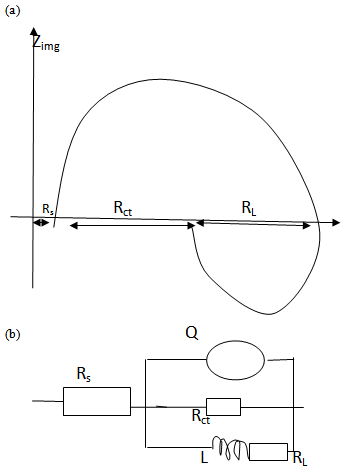 | Figure 6. The complex impedance diagram (a) and the equivalent circuit diagram for total impedance (b) for the theoretical calculations |
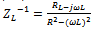 | (4) |
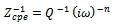 | (5) |
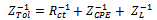 | (6) |
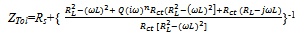 | (7) |
 From the results presented in table 2, the effects of heat treatment are very pronounced, the values of CPE Q decreased, and oven quenched being the least. The non- ideality in capacitive behavior n changed in the heat treated specimen, with air quenched deviating most. Other parameters Rct, RL, and L, all increased in the heat treated samples, with the air quenched sample being higher in each case. These high resistance values give credence to the low current densities observed in the potentiodynamic polarization. The capacitance at maximum imaginary impedance determined by
From the results presented in table 2, the effects of heat treatment are very pronounced, the values of CPE Q decreased, and oven quenched being the least. The non- ideality in capacitive behavior n changed in the heat treated specimen, with air quenched deviating most. Other parameters Rct, RL, and L, all increased in the heat treated samples, with the air quenched sample being higher in each case. These high resistance values give credence to the low current densities observed in the potentiodynamic polarization. The capacitance at maximum imaginary impedance determined by 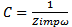 | (8) |
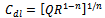 | (9) |
4. Conclusions
- The electrochemical behavior of unheated and heated specimens of aluminium alloy AA8011 in 0.1M H2SO4 acid environment were investigated after the XRD and SEM measurements of the samples were taken.- The XRD profiles showed, sharp, narrow, high peak values for all the alloy samples, showing high crystallinity of the AA8011 alloy sample.- The heat treatment did not caused cause increase splitting of the crystallites but caused the formation of less harmful crystallite Al6(FeMn) for air quenched sample and increase in the crystallite FeMn in the oven quenched .- The SEM data revealed a decrease in the size number and grain volume fraction of the crystallites.- The reduction in the size and grain volume fraction observed in the heat treated specimens translated to increase in corrosion resistance.- The OCP, PP, and EIS all buttress the effect of heat treatment on the corrosion resistance of the AA8011 in the acid environment; this is shown in lower current densities, higher capacitive loops and larger values of simulated parameters.- The lower values of double layer capacitance and higher thickness of the oxide layer might have contributed immensely to the blockage of active sites.- The information garnered from this research forms and opens a window of application of this alloy in service environment.
References
| [1] | Tierce, S, Pebere, N, Blanc, C, Casenave, C, Mankoiski, G, Robidou, H (2006) Corrosion behaviour of brazing material AA4343. Electorchim Acta, vol 53, 3, 12 p 1092. |
| [2] | Wang, S.S, Cheng, M.D, Tsao, L.C, Chuang, T.H, (2001) Corrosion behaviours of Al-Si-Cu (Sn, Zn) brazing filler metals mat chara 47, 4014 |
| [3] | Wang, G and Jiao, H, (2010), Microstructural Effects in Corrosion of Aluminium tube alloys, Trans. Nonferrous Met. Soc. China 21, 1193-1198. |
| [4] | Goncalves, W.F.O, Zoqui, E.J, Paes, M,(2006) Effect of Grain refining and Homogenizing treatment on Al-Fe-Mn-Si cast alloys, 17th CBECIMat- Congresso Brasileiro de Engenharia e Clencia dos materials, 5080-5088. |
| [5] | Delijic, K, Asanovic, V, Radonjic, D, Mechanical and corrosion properties of Aa8011 sheets and foils, Mat. Tech. 40(3) 83-88. |
| [6] | Borodiak, M, Pinheiro, F.P, and Pacs, Marcedo, (2012) Metallurgical characterization of Aluminium alloy by matrix dissolution, Light metal 2012,Ed. Carlou E.S, TMS. |
| [7] | Ney, J and Luiggi, A (1998) Charaterization by thermoelectric power of commercial aluminium- Iron- silicon alloy AA8011 during isothermal precipitation, Metall. Mater. Trans. 19A, 2669. |
| [8] | Oscarson , A, Lehtinen, B, Hutchinson, B, Ekstron, H. E, Bate, P, Haggstron, L, Ghandour, A.M (1994) in preceeding of the 4th International Conference on Aluminum alloys. Altlanta. Georgia, USA Vol. III, 144. |
| [9] | Anderson , B, Naess, S.E. (1987) in Proceedings of the 8th International Light Metals Congress, Leoben. Viena, (Aluminium Verglag GmbH), 526 |
| [10] | Marshall, G.J, Bolingbroke, R.K, Gray, A (1993) Microstructural control in aluminium core alloy for brazing sheet applications, Metallurg. Mat. Trans. A 1935-1942. |
| [11] | Howe, J.M.(1986) Metallographical and differential scanning calorimetry analyses of precipitation and crystallization in an Al-Mn alloy, Metall. Trans. A. Vol. 17, Iss. 4, pp593-605. |
| [12] | Bahadur, A (1988), Intermetallic phases in Al-Mn alloys, Journ. of Mater. Sci. 23, 48-54. |
| [13] | Li, Y.J, Arnberg, L, (2003) Evolution of eutectic intermetallic particles in DC-cast AA3003 alloy during heating and homogenization, Mater. Sci. and Eng. A, 347. |
| [14] | Slamova, M, Ocenasek, V, Cieslar, M, Chalupa, B, Merle, P (2000) “Differences in structure evolution of twin roll cast AA8006 and AA8011 during annealing”. Mater. Sci. Forum Vol. 331-337, 829-834. |
| [15] | Cullity, B.D, Element of X-Ray Diffraction, 2nd ed. 1972, Addison-Wesley, Pub. Coy, California, USA. |
| [16] | Ruhi,G, Modi, O.P, Jha, A.K, Singh, I.B, (2009) Characterization of corrosion resistance properties of sol-gel aluminium coating in marine water environment , Ind. Journ. Chem. Techn. Vol.16, 216-220 |
| [17] | Yanjun, L, Amberg, L (2003), “Precipitation of dispersoids in DC cast AA3103 alloy during heat treatment” Light metal 2003, 991-997. |
| [18] | Szklarska- Smialowska, Z, (1999), Pitting Corrosion of aluminium, corros.Sci. 41, 1743- 1767. |
| [19] | Birbilis, N, Buchheit, R.G, (2005) “Electrochemical Characteristics of Intermetallic Phases in Aluminium Alloys: An Experimental Survey and Discussion” Journ. Electrochem. Soc. 152 (4) B140-B151. |
| [20] | Ambat , R, Davenport, A.J, Scamans, G.M, Afseth, A, (2005) Electrochemical behaviour of the active surface layer on rolled aluminium alloy sheet, J. Electrochem. Soc. B, 151(1),53-58. |
| [21] | Liu, Y, Chen, Y. F, (2010), The role of second phase particles in pitting corrosion ofAL alloy in NaCl solution, mater. And corros, vol.61 iss 3, 211-217. |
| [22] | Metikos-Hukovic, M, Babic, R, Grubac, Z,(2002) The study of aluminium corrosion in acidic solution with nontoxic inhibitors, Journ. of Appl. Electrochem. 32, 35-41. |
| [23] | Osorio, W.R., Cremasco, A, Andrade, P.N, Carcia, A. Caram, (2010), Electrochemical behavior of centrifuged cast and heat treated Ti-Cu alloys for medical applications. Electochimica Acta 55, 759-770. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML