-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2012; 2(4): 158-164
doi: 10.5923/j.ijmc.20120204.08
Corrosion Inhibition of Mild Steel in Hydrochloric Acid by Acid Extracts of Eichhornia Crassipes
S. B. Ulaeto 1, 2, 3, U. J. Ekpe 1, M. A. Chidiebere 3, E. E. Oguzie 3
1Corrosion and Electrochemistry Research Group, Department of Pure and Applied Chemistry, University of Calabar, PMB 1115, Calabar, Nigeria
2Department of Physical and Chemical Sciences, Rhema University, Aba, Abia State, Nigeria
3Electrochemistry and Material Science Research Laboratory, Department of Chemistry, Federal University of Technology, PMB 1526, Owerri, Nigeria
Correspondence to: U. J. Ekpe , Corrosion and Electrochemistry Research Group, Department of Pure and Applied Chemistry, University of Calabar, PMB 1115, Calabar, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Acid extracts from leaves and roots of Eichhornia crassipes (water hyacinth) were tested as corrosion inhibitors for mild steel in hydrochloric acid solutions using a gasometric technique. The effects of temperature and concentration on the inhibition performance of the extracts have been studied. The results show that both the leaf and root extracts functioned as effective corrosion inhibitors, with the leaf extracts exerting a greater effect. Fitting of the experimental data to the Arrhenius and transition state equations revealed that the organic constituents of the extracts were physically adsorbed on the corroding mild steel surface. The adsorption characteristics of selected extract constituents were theoretically evaluated by molecular dynamics simulations in the framework of the density functional theory and confirm distinct adsorption of the extract organic matter on the mild steel surface. Our findings provide ready eco-friendly application for the problematic fresh water weed Eichhornia crassipes.
Keywords: Acid Corrosion Inhibition, Gasometric Technique, Mild Steel, Eichhornia Crassipes, Molecular Dynamics Simulation
Cite this paper: S. B. Ulaeto , U. J. Ekpe , M. A. Chidiebere , E. E. Oguzie , "Corrosion Inhibition of Mild Steel in Hydrochloric Acid by Acid Extracts of Eichhornia Crassipes", International Journal of Materials and Chemistry, Vol. 2 No. 4, 2012, pp. 158-164. doi: 10.5923/j.ijmc.20120204.08.
Article Outline
1. Introduction
- Iron and its alloys are used as materials of choice in diverse industrial and structural applications. Acid solutions are often used in drilling operations in oil and gas exploration, as well as for cleaning, decaling and pickling of steel structure; processes which are normally accompanied by considerable dissolution of the metal and it is very important to add corrosion inhibitors to decrease the corrosion rate in such situations. Organic heterogeneous compounds containing these elements have been reported to be efficient corrosion inhibitors[1-8]. These compounds contain nitrogen, oxygen, sulphur and aromatic ring in their molecular structures and function via adsorption of the molecules on the metal surface creating a barrier to corrodent attack. The use of natural products of plant origin as corrosion inhibitors has been widely reported by several authors[9-19]. Such interest derives from their inexpensive and eco-friendly nature, easy availability and wide variety. Also the use of these biomass products is justified by the phytochemical compounds present therein with molecular and electronic structures bearing close similarity to conventional organic inhibitor molecules[11]. The yield of these compounds as well as the corrosion inhibition abilities vary widely depending on the part of the plant and its geographical location[13]. E. crassipes is noted as one of the most important and noxious freshwater weeds[20], ranked 8th in the list of the worlds ten most serious weeds according to[21]. It is a floating aquatic plant with inflated petioles and native to tropical America[22]. It is believed that the weed originated in Brazil[23] but found its way into Nigerian waters from a lagoon in the Port Novo area of the Republic of Benin which opens into the Badagry creek enroute to the Atlantic ocean [22]. Since then Eichhornia crassipes has become a major weed in Nigeria, having successfully invaded and established itself on the entire Badagary Creek, the Yewa Lagoon, Ologe Lagoon, the Lagos Lagoon and the waterways of Okitipupa, it was reported in 1982 in local newspapers as a weed that is spreading fast and paralyzing the fishing industry[22]. Despite its long list of harmful effects, in recent years it has been found useful in animal feeds, compost, paper, energy (from biogas), biological waste water treatment and heavy metals uptake[20]. In recent times, studies have been carried out to identify more useful applications of this abundant noxious weed and certain active compounds with antioxidant activities such as chlorophylls and carotenoids, phenols, alkaloids and terpenoids have been successfully obtained from E. Crassipes extracts[24]. The antioxidants showed corrosion inhibition efficiency on magnesium alloy and it may be due to the presence of a great number of double bonds, amine and hydroxyl groups known for their oxygen scavenging and hydrogen donating antioxidant activities[24]. However, to the best of our knowledge, the plant has not been studied for corrosion inhibition abilities on mild steel. The present study investigates the inhibitive effect of leaves (ELV) and roots (ERT) extracts of Eichhornia crassipes on mild steel corrosion in HCl solutions using gasometric technique and its modelled structures provides additional insight into the mechanism of inhibitory action.
2. Materials and Methods
2.1. Metal Specimen
- The mild steel sheets used in this present work have the composition presented in Table 1. Before measurements, the mild steel coupons were mechanically polished with series of emery paper of variable grades starting with the coarsest and proceeding in steps to the finest (1200) grade, degreased with absolute ethanol, dipped into acetone and air dried. All experiments were conducted on mild steel coupons of dimension 2.0 x 0.08 x 5.0 cm (with a surface area of 21.12 cm2).
|
2.2. Preparation of Plant Extracts
- Leaves and roots of E. crassipes were collected from a water dam in the University of Ibadan, Nigeria. They were cleaned from epiphytes, washed, dried to a constant weight, ground and kept in labelled glass jars till use. Stock solutions of the leaves and roots extracts were prepared by soaking 4.0 g of the dried and ground leaves and roots in 1000 ml of 5 M HCl solution. The resultant solution was kept for 24 hours, filtered and stored. From the stock solution (4.0 g/l), inhibitor test solutions (concentrations of 0.1, 0.5, 1.0 and 2.0g/l) were prepared.
2.3. Gasometric Experiments
- Gasometric measurements were carried out as previously described[25, 26]. Experiments were conducted at 30, 40, 50 and 60℃. Gasometric technique is based on the principle that corrosion reactions in aqueous acidic media are characterized by the evolution of gas resulting from the cathodic reaction of the corrosion process, which is proportional to the rate of corrosion[27]. The rate of evolution of the gas (RH) is determined from the slope of the graph of volume of gas evolved (V) versus time (t) and the degree of surface coverage (θ) and hence inhibition efficiency (η%) determined using equations (1) and (2), respectively.
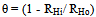 | (1) |
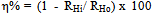 | (2) |
2.4. Quantum Chemical Calculations
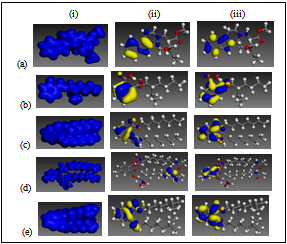
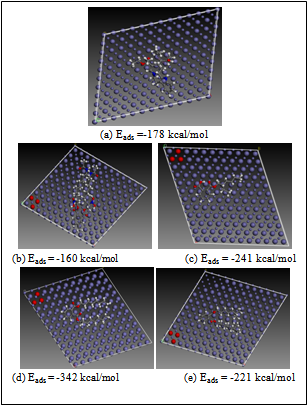
- All theoretical calculations were performed using the density functional theory (DFT) electronic structure programs Forcite and DMol3 as contained in the Materials Studio 4.0 software (Accelrys, Inc.). We modeled the molecular electronic structures of the compounds, including the distribution of frontier molecular orbitals and Fukui indices in order to establish the active sites as well as the local reactivity of the molecules. The calculations were performed by means of the DFT electronic structure program DMol3 using a Mulliken population analysis[30,31]. Electronic parameters for the simulation include restricted spin polarization using the DND basis set and the Perdew Wang (PW) local correlation density functional. Geometry optimization was achieved using COMPASS force field and the Smart minimize method by high-convergence criteria.Forcite quench molecular dynamics was used to sample many different low energy adsorption configurations of the different molecules on Fe[32,33]. The Fe crystal was cleaved along the (110) plane. Calculations were carried out in a 12 x 8 supercell using the COMPASS force field and the Smart algorithm with NVE (microcanonical) ensemble, a time step of 1 fs and simulation time 5 ps. Temperature was fixed at 350 K.
3. Results and Discussion
- We have employed gas-volumetric measurements to investigate mild steel corrosion in 5 M HCl solutions in the absence and presence of ELV and ERT extracts, which are studied herein for corrosion-inhibiting efficacy.
3.1. Corrosion Rates
- Figure 1 shows the representative hydrogen evolution plots for mild steel in uninhibited 5 M HCl at 30 – 60℃. The data presented are means of triplicate determinations, with standard deviation ranging from 0 to 0.003. Hydrogen gas evolution can be seen to increase with increase in temperature resulting in degradation of the mild steel.Figure 2 illustrates the hydrogen gas evolution rates of the mild steel specimen in 5 M HCl in the presence of different concentrations of ELV (Figure 2a) and ERT (Figure 2b) at different temperatures. The plots indicate that the extracts actually retarded mild steel corrosion at all concentrations in 5 M HCl and the inhibiting effect becomes more pronounced at higher extract concentrations. The data in Figures 1 and 2 also indicate that the rates of steel corrosion in absence and presence of the extracts increased with rise in temperature. This is because an increase in temperature usually accelerates corrosive processes, particularly in media in which H2 gas evolution accompanies corrosion, giving rise to higher dissolution rates of the metal.
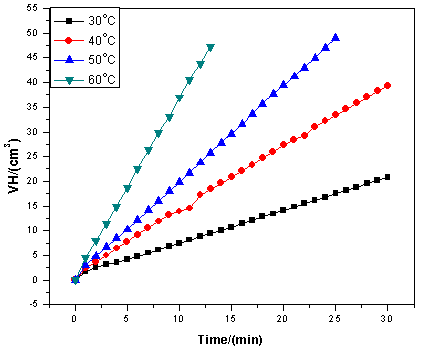 | Figure 1. Hydrogen evolution plots for mild steel in uninhibited 5 M HCl at 30 – 60℃ |
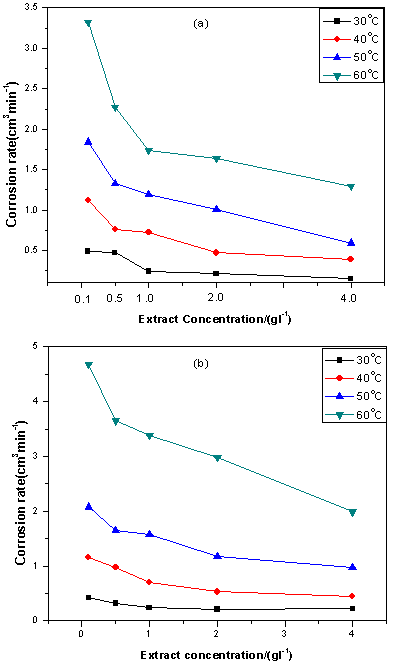 | Figure. 2. Variation of corrosion rates with extracts concentration for mild steel in 5 M HCl containing (a) ELV and (b) ERT |
3.2. Inhibition Efficiency and Adsorption Considerations
- Quantitative characterization of the inhibiting effect of PNG extract on the free corrosion of mild steel was carried out by an assessment of the inhibition efficiency (IE %) defined by Eq. 2 above. Figures 3a and 3b show the variation of inhibition efficiency with extract concentration and temperature in 5 M HCl. Inhibition efficiency increased steadily with increasing extract concentration and decreases with rise in temperature. The increase in efficiency of inhibition with extract concentration indicates that more of the extract constituents are adsorbed on the metal surface at higher concentration, leading to greater surface coverage. Declining efficiency with rise in temperature suggests a possible shift of the adsorption–desorption equilibrium towards desorption of adsorbed inhibiting species, since the interface becomes increasingly agitated due to higher rates of hydrogen gas evolution, thus perturbing the adsorbed species. Additionally, the roughening of the metal surface as a result of enhanced corrosion could also reduce the ability of the inhibitor to be adsorbed on the metal surface at high temperatures.
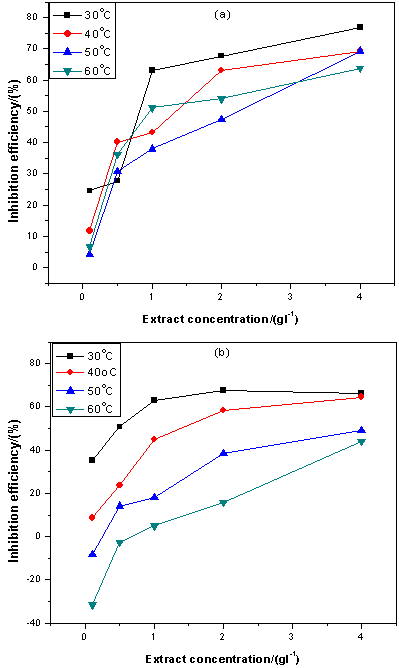 | Figure 3. Variation of inhibition efficiency with extracts concentration for mild steel in 5 M HCl containing (a) ELV (b) ERT |
3.3. Thermodynamics Parameters
- The Arrhenius-type relationship between the corrosion rate (k) of mild steel in acidic media and temperature (T) as often expressed by the Arrhenius equation was used to determine the activation energies (Ea):
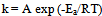 | (3) |
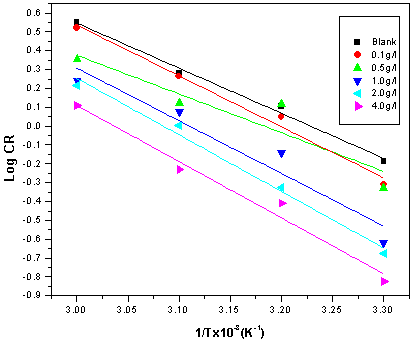 | Figure 6. Arrhenius plots for mild steel corrosion in 5 M HCl in the absence and presence of different concentrations of ELV |
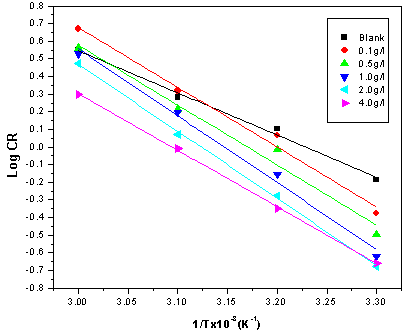 | Figure 7. Arrhenius plots for mild steel corrosion in 5 M HCl in the absence and presence of different concentrations of ERT |
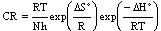 | (4) |
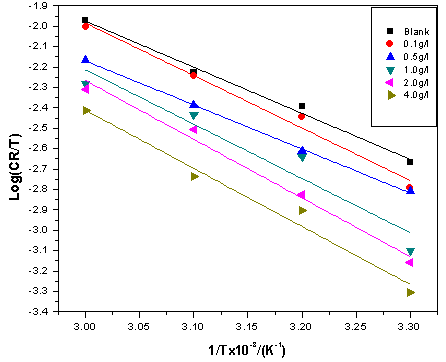 | Figure 8. Eyring plots for mild steel corrosion in 5 M HCl without and with ELV |
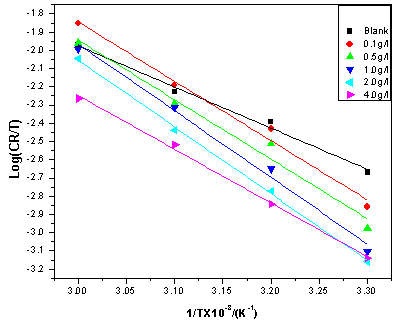 | Figure 9. Eyring plots for mild steel corrosion in 5 M HCl without and with ERT |
4. Conclusions
- The aim of this research was to determine the feasibility of exploiting the bothersome weed (Eichhornia crassipes ) for materials corrosion control. Our findings show that the leaf (ELV) and root extracts (ERT) of E. crassipes effectively inhibited mild steel corrosion in 5 M HCl. At higher concentrations and lower temperatures, the extracts performed better, whereas at low concentrations and high temperature, their inhibition efficiency decreased. ELV inhibited better than ERT at ordinary temperature and highest concentration of 4 g/l. The inhibiting potential of the extracts were theoretically confirmed via, DFT based quantum chemical computations of parameters associated with the electronic and adsorption structures of selected phytochemical components of the extract. These results propose a ready industrial application for the problematic fresh water weed for the control of the acid corrosion of mild steel.
ACKNOWLEDGEMENTS
- The authors acknowledge Patrick Asuquo for technical assistance in performing some measurements
References
| [1] | V. U. Khuzhaeu, S. F. Aripova (2004) Chem. of Nat. Comp, 36(4): 418 |
| [2] | U. Ogan (1971) Phytochem. Rep., 99(2/3): 441 |
| [3] | U. J. Ekpe, P.C. Okafor, E.E. Ebenso, O.E. Offiong, B.I Ita (2001) Bull. of Electrochem., 17: 131 |
| [4] | E. E. Ebenso, P.C. Okafor, U.J. Ekpe, U.J. Ibok, A.I. Onuchukwu, J. (2004) Chem. Soc. Nigeria, 29(1): 15 |
| [5] | P. C. Okafor, E.E. Ebenso, U.J. Ibok, U.J. Ekpe, M.I. Ikpi (2003) Trans. SAEST, 38: 91 |
| [6] | K. F. Khaled, N. Hackerman (2003) Mater. Chem. Phys., 82: 949 |
| [7] | P. C. Okafor, E.E. Ebenso, U.J. Ekpe (2004) Bull. Chem. Soc. Ethiopia, 18: 181 |
| [8] | E. E. Ebenso, E.E. Oguzie (2005) Mater. Lett. 59(17): 2163 |
| [9] | F. Zucchi, H. I. Omar (1985) Surface Tech., 24(4): 391 |
| [10] | S. P. Ramesh, K. V. Kumar, M. G. Sethuraman (2001) Bull. of Electrochem., 17(3): 141 |
| [11] | P.C. Okafor, U.J. Ekpe, E.E. Ebenso, E.E. Oguzie, N.S. Umo, A.R. Etor (2006) Trans. SAEST, 41: 82 |
| [12] | M. Abdel-Gaber, B. A. Abd-El-Nabel, I. M. Sidahmed, A. M. El-Zayady, M. Saadawy (2006) Corros. Sci., 48: 765 |
| [13] | E.E. Oguzie (2006) Pigm. Res. Tech., 35(2): 63 |
| [14] | P. C. Okafor, E. E. Ebenso (2007) Pigm. Res. Tech., 36(3): 134 |
| [15] | P. C. Okafor, V. I. Osabor, E. E. Ebenso (2007) Pigm. Res. Tech., 36(5): 299 |
| [16] | E. E. Oguzie (2008) Corros. Sci.50: 2993-2998 |
| [17] | P. C. Okafor, M. E. Ikpi, I.E., Uwah, E.E., Ebenso, U.J Ekpe, S.A. Umoren (2008) Corros. Sci., 50: 2310 |
| [18] | P.C. Okafor, I.E. Uwah, O.O. Ekerenam, U.J. Ekpe (2009) Pig. Res. Tech., 38(4): 236 |
| [19] | E. E. Oguzie, C. K. Enenebeaku, C. O Akalezi, S. C. Okoro, A. A. Ayuk, E. N. Ejike, J. (2010) Colloid Interf. Sci., 349: 283 |
| [20] | B. Gopal (1987) Water Hyacinth: Aquatic plant studies 1. New York: Elsevier Science Publishing Company. |
| [21] | K. R. Reddy, D. L. Sutton (1984) J. of Environ. Quality, 13: 1 |
| [22] | O. A. Akinyemiju (1987) J. of Aquatic Plant Mgt., 25: 24 |
| [23] | T. D. Center, M. P. Hill, H. Cordo, M. H. Julien (2002) USDA Forest Service Publication FHTET-2002-04: 41 |
| [24] | S. M. M. Shanab, M. A. Ameer, A. M. Fekry, A. A. Ghoneim and E. A. Shalaby (2011) Int. J. Electrochem. Sci.,6: 3017 |
| [25] | U. J. Ekpe, U.J. Ibok, B.I. Ita, O.E. Offiong, E.E. Ebenso (1995) Mater. Chem. Phys., 40(2): 87 |
| [26] | B. I. Ita, O.E. Offiong (1997) Mater. Chem. Phys., 48(2): 164 |
| [27] | P.C. Okafor, U.J. Ekpe, E.E. Ebenso, E.M Umoren, K.E. Leizou (2005) Bull. Electrochem., 21(8): 347 |
| [28] | A.Y. El-Etre (2003) Corros. Sci., 45: 2485 |
| [29] | M. Abdallah (2004) Corros. Sci., 46(8): 1981. |
| [30] | B.J. Delley (1990) Chem. Phys., 92: 508. |
| [31] | B.J. Delley (2000) Chem. Phys., 113: 7756. |
| [32] | C.J. Casewit, K.S. Colwell, A.K. Rappé (1992) J. Am. Chem. Soc. 114: 10035. |
| [33] | C.J. Casewit, K.S. Colwell, A.K. Rappé (1992) J. Am. Chem. Soc. 114: 10046. |
| [34] | D. Turcio-Ortega, T. Pandiyan, J. Cruz, E. Garcia-Ochoa (2007) J. Phys. Chem. C. 111: 9853. |
| [35] | G. Gece (2008) Corros. Sci., 52: 2981. |
| [36] | I.B. Obot, N.O. Obi-Egbedi (2010) Corr. Sci. 52: 198. |
| [37] | K.F. Khaled (2008) Electrochim. Acta, 53: 3484. |
| [38] | E.E. Oguzie, Y. Li, S.G. Wang, F.H. Wang (2011) RSC Advances, 1: 866. |
| [39] | S. Matai, D. K. Bagchi, (1980) 144-148. In A. Granam, S. I. Krishaswamy, J. S. Kaha, (eds.). Proceedings International Symposium on Biological Applications of Solar Energy. Mandras: Macmillan Company of India. |
| [40] | S. A. Umoren, I. B. Obot, E. E. Ebenso, P. C. Okafor, O. Ogbobe, E. E. Oguzie (2006) Anti-Corros. Methods & Mater., 53(5): 277 |
| [41] | E. E. Ebenso, U. J. Ekpe (1996) West African Journal of Biological and Applied Chem., 41: 21 |
| [42] | M. A. Ameer, E. Khamis, G. Al-Sanani (2000) Adsorp. Sci. & Technol., 18: 177 |
| [43] | E. E. Ebenso, U. J. Ekpe,S. Umoren, J. Ekerete,O. K. Abiola, S. Martinez (2004) J. Corros. Sci. Technol., 1: 96 |
| [44] | E. E. Ebenso, N. O. Eddy, A. O Odiongenyi (2009) Portugaliae Electrochim Acta., 27: 13 |
| [45] | E. E. Oguzie, B. N. Okolue, E. E. Ebenso, G. N. Onuoha, A. I. Onuchukwu (2004) Maters. Chem. Phy., 87: 394 |
| [46] | S. A. Umoren, O. Ogbobe, P. C. Osabor, E. E. Ebenso (2007) J. of Applied Polymer Sci., 105: 3363 |
| [47] | E.E. Oguzie, K.I. Iyeh and A.I. Onuchukwu (2006) Bull. Electrochem., 22(2): 63 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML