-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2012; 2(4): 141-144
doi: 10.5923/j.ijmc.20120204.05
Synthesis and Structural Characterization of (S)-Tetrahydro-Pyrrol-[1,2,c]-Imidazole-1,3-Dione
Gerzon E. Delgado , Jines E. Contreras
Laboratorio de Cristalografía, Departamento de Química, Facultad de Ciencias, Universidad de Los Andes, Mérida 5101, Venezuela
Correspondence to: Gerzon E. Delgado , Laboratorio de Cristalografía, Departamento de Química, Facultad de Ciencias, Universidad de Los Andes, Mérida 5101, Venezuela.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this work we present the synthesis and X-ray single crystal structural characterization of the heterocyclic compound (S)-tetrahydro-pyrrol-[1,2,c]-imidazole-1,3-dione. This material crystallize in the orthorhombic system with space group P212121 (Nº19), Z=4, and unit cell parameters a = 7.136(1) Å, b = 8.009(2) Å, c = 11.378(2) Å. The molecular structure shows a hydantoin and pyrrolidine ring coupling forming a bicyclohydantoin. The crystal packing is governed by N--H···O hydrogen bond-type intermolecular interactions, forming infinite one-dimensional chains.
Keywords: Hydantoin, Hydrogen Bonding, X-ray Crystal Structure
Cite this paper: Gerzon E. Delgado , Jines E. Contreras , "Synthesis and Structural Characterization of (S)-Tetrahydro-Pyrrol-[1,2,c]-Imidazole-1,3-Dione", International Journal of Materials and Chemistry, Vol. 2 No. 4, 2012, pp. 141-144. doi: 10.5923/j.ijmc.20120204.05.
Article Outline
1. Introduction
- The imidazolidine-2,4-dione, or hydantoin, is a common 5-member ring containing a reactive cyclic urea core[1,2]. This heterocycle represents a significant molecular template in combinatorial chemistry libraries[3-5], due principally to the four possible points of substitutions. The biological activities of hydantoin derivatives has been known for a long time, and are responsible for a wide variety of biological behavior[6], due principally to its wide range of therapeutic properties. For instance, several applications have been reported for hydantoins: antiarrhythmic andantihypertensive[7], antiviral[8], antineoplastic[9], antitumoral[10] and anticonvulsant agents[11]. The best knwon hydantoin, phenytoin, is the most widely used antiepileptic drug[12]. In addition, these compounds are used as herbicides[13] and fungicides agents[14]. On the other hand, the biocatalytic conversion of 5-subtituted hydantoins to amino acids has received considerable attention recently for their potential applications in the industrial productions of optically pure amino acids[15,16].For these reasons, there has been much interest in the search of new synthetic routes for hydantoin via solution[17], or solid state reactions[18-21].In our laboratory we are interested in the study of N-carbamoyl and hydantoin natural amino acids derivative compounds[22-26], therefore we report here the structure of (S)-tetrahydro-pyrrol-[1,2,c]-imidazole-1,3-dione, the hydantoin derivative of the natural amino acid L-proline. The analysis of the hydrogen bond patterns is also discussed.
2. Experimental
2.1. Synthesis
- The title compound was synthesized from L-proline using a methodology previously reported[22,23]. 500 mg (4.3 mmol) of L-proline was disolved in 20 mL of water and the solution was acidified with concentrated HCl (37 % v/v) to pH = 5. Then, 1050 mg (12.9 mmol) of KOCN was added to this solution. The mixture was warmed up, with agitation, to 60 ℃, during 4 h. The resultant solution was acidified with HCl to pH = 2 and agitated during 4 h, until the precipitation of a white solid. (see scheme 1). The solid was filtered and washed with cool water. Colorless crystals of 1 suitable for X-ray diffraction analysis were grown by slow evaporation in a 1:1 methanol-water solution (m.p.: 210-212 ℃). FT-IR 1757.6 cm-1 [t, C=O], 1708.7 cm-1 [t, C=O]. 1H NMR (400 MHz, DMSO-d6) δ =7.27 (H3), 4.07 (t, H5), 3.43 (q, H6A), 3.02 (q, H6B), 2.03 (s, H8A), 1.60 (s, H8B), 1.90 (m, H7A), 1.93 (H7B). 13C NMR (100.6 MHz, DMSO-d6) δ =161.0 (C2), 174.5 (C4), 64.0 (C5), 44.9 (C6), 26.7 (C8), 26.6 (C7).
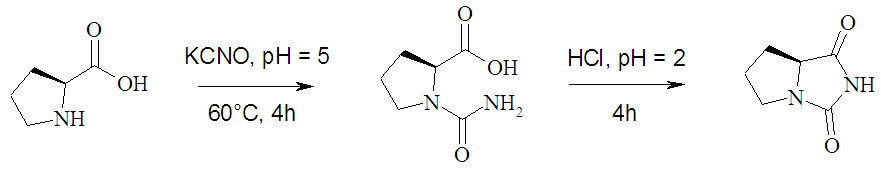 | Scheme 1. Synthesis of (S)-tetrahydro-pyrrol-[1,2,c]-imidazole-1,3-dione |
2.2. X-ray crystallography
- Colorless rectangular crystal (0.4, 0.2, 0.1 mm) was used for data collection. Diffraction data were collected at 298(2) K by ω-scan technique on a Rigaku AFC7S Mercury diffractometer [27] equipped with graphite-monochromatized MoKα radiation (λ = 0.71073 Å). The data were corrected for Lorentz-polarization and absorption effects[28]. The structure was solved by direct methods using the SHELXS program[29] and refined by a full-matrix least-squares calculation on F2 using SHELXL[28]. The absolute structure was assigned from the known configuration of L-proline.All H atoms were placed at calculated positions and treated using a riding model, with C-H distances 0.96-0.98 Å and Uiso(H) = 1.2Ueq(C)], N-H 0.86 Å and Uiso(H) = 1.2Ueq(N)].
3. Results and discussion
- Figure 1 shows the molecular structure and the atom-labeling scheme of 1[30], and Table 1 shows the crystallographic data and structure refinement parameters.
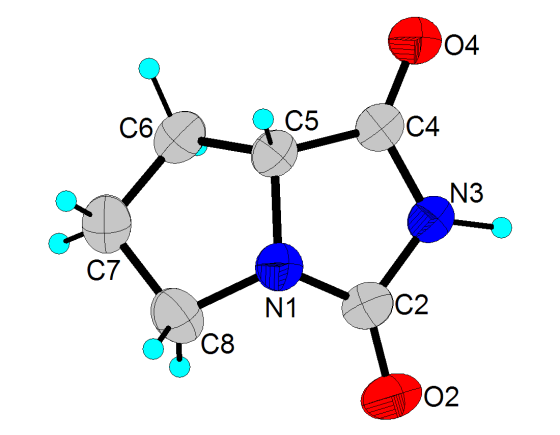 | Figure 1. The molecular structure of 1, showing the atomic numbering scheme. Displacement ellipsoids are drawn at 50% probability level. H atoms are shown as spheres of arbitrary radii |
|
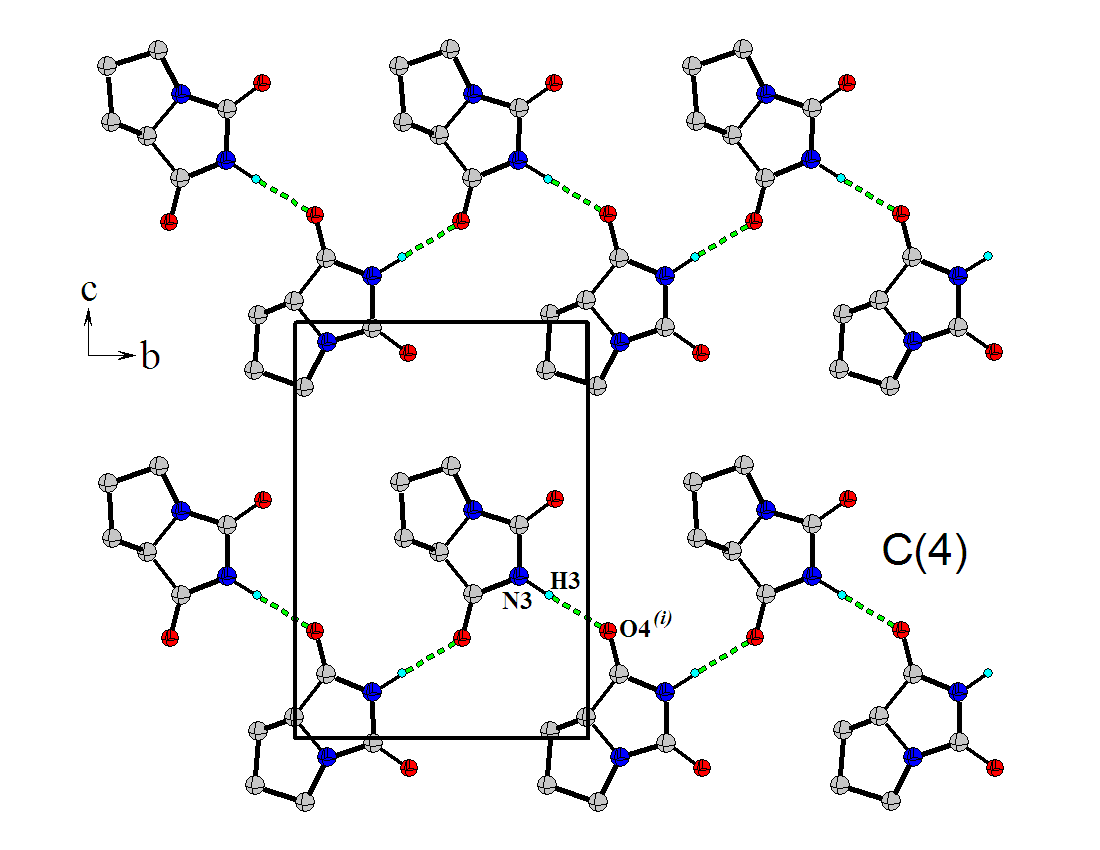 | Figure 2. A portion of the crystal packing viewed in the cb plane. Intermolecular hydrogen bonds, N--H···O, are indicated by dashed lines. H atoms not involved in hydrogen bonding have been omitted for clarity |
|
4. Conclusions
- In the crystal structure of (S)-tetrahydro-pyrrol-[1,2,c]- imidazole-1,3-dione, the molecules are linked by N---H···O hydrogen bonds, forming infinite one-dimensional zigzag chains, running along [010] plane, with a C(4) graph-set motif.
ACKNOWLEDGEMENTS
- This work was supported by CDCHT-ULA (grant C-1755-11-08-B) and FONACIT (grant LAB-97000821).
References
| [1] | C.A. López, G.G. Trigo, “The chemistry of hydantoins”, Adv. Heterocycl. Chem. 38, 177-210, 1985. |
| [2] | M. Meusel, M. Gütschow, “Recent developments in hydantoin chemistry. A review”, Org. Prep. Proced. Int. 36, 391-443, 2004. |
| [3] | K.H. Park, J. Ehrler, H. Spoerri, M.J. Kurth, “Preparation of a 990-member chemical compound library of hydantoin- and isoxazoline-containing heterocycles using multipin technology”, J. Comb. Chem. 3, 171-176, 2001. |
| [4] | M.J. Lin, C.M. Sun, “Microwave-assisted traceless synthesis of thiohydantoin”, Tetrahedron Lett. 44, 8739-8742, 2003. |
| [5] | W. Zhang, Y.M. Lu, C.H.T Chen, L. Zeng, D.B. Kassel, “Fluorous mixture synthesis of two libraries with hydantoin-, and benzodiazepinedione-fused heterocyclic scaffolds”, J. Comb. Chem. 8, 687-695, 2006. |
| [6] | E. Mutschler, H. Derendorf, “Drug Actions, Basic Principles and Therapeutic Aspects”, Medpharm Scientific Publishers, Stuttgart, 1995. |
| [7] | T. Dylag, M. Zygmunt, D. Maciag, J. Handzlik, M. Bednarski, B. Filipek, K. Kiec-Kononowicz, “Synthesis and evaluation of in vivo activity of diphenylhydantoin basic derivatives”. Eur. J. Med. Chem. 39, 1013-1027, 2004. |
| [8] | N. Opacic, M. Barbarić, B. Zorc, M. Cetina, A. Nagl, D. Frkovic, M. Kralj, K. Pavelic, J. Balzarini, G. Andrei, R. Snoeck, E. De Clercq, S. Raić-Malić, M. Mintas, “The novel L- and D-Amino acid derivatives of hydroxyurea and hydantoins: Synthesis, X-ray crystal structure study, and cytostatic and antiviral activity evaluations,” J. Med. Chem. 48, 475-482, 2005. |
| [9] | E. Lattmann, W.O. Ayuko, D. Kinchinaton, C.A. Langley, H. Singh, L. Karimi, M.J. Tisdale, “Synthesis and evaluation of 5-arylated 2(5H)-furanones and 2-arylated pyridazin-3(2H)-ones as anti-cancer agents”, J. Phar. Pharmacol. 55, 1259-1265, 2003. |
| [10] | C. Carmi, A. Cavazzoni, V. Zuliani, A. Lodola, F. Bordi, P.V. Plazzi, R.R. Alfieri, P.G. Petronini, M. Mor, “5-Benzylidene-hydantoins as new EGFR inhibitors with antiproliferative activity”, Bioorg. Med. Chem. Lett. 16, 4021-4025, 2006. |
| [11] | G. Singh, P.H. Driever, J.W. Sander, L. Sander, “Cancer risk in people with epilepsy: The role of antiepileptic drugs”, Brain 128, 7-17, 2005. |
| [12] | H.H. Merrit, T.J. Putnam, “A new series of anticonvulsant drugs tested by experiments on animals”, Arch. Neurol. Psychiatry 39, 1003-1015, 1938. |
| [13] | M. Shiozaki, “Syntheses of hydantocidin and C-2-thioxohydantocidin”, Carbohydr. Res. 337, 2077-2088, 2002. |
| [14] | J. Marton, J. Enisz, S. Hosztafi, T. Timar, “Preparation and fungicidal activity of 5-substituted hydantoins and their 2-thio analogs,” J. Agric. Food. Chem. 41, 148-152, 1993. |
| [15] | B. Wilms, A. Wiese, C. Syldatk, R. Mattes, J. Altenbuchner, “Development of an escherichia coli whole cell biocatalyst for the production of L-amino acids”, J. Biotechnol. 86. 19-30, 2001. |
| [16] | S.G. Burton, R.A. Dorrington, “Hydantoin-hydrolysing enzymes for the enantioselective production of amino acids: new insights and applications”, Tetrahedron Asymm. 15, 2737-2741, 2004. |
| [17] | E. Kleinpeter, “The structure of hydantoins in solution and in the solid state”, Struct. Chem. 2, 161-173, 1997. |
| [18] | A. Ganesan, “Cyclative cleavage strategies for the solid-phase synthesis of heterocycles and natural products”, Methods Enzymol. 369, 415-434, 2003. |
| [19] | J. Vázquez, M. Royo, F. Albericio, “Re-evaluation of a solid-phase hydantoin synthesis”. Lett. Org. Chem. 1, 224-226, 2004. |
| [20] | J. Alsina, W.L.Scott, M.J. O’Donnell, “Solid-phase synthesis of α-substituted proline hydantoins and analogs”, Tetrahedron Lett. 46, 3131-3135, 2005. |
| [21] | V. Kumar, H. Rana, R. Sankolli, M.P. Kaushik, “Highly efficient dialkylphosphate-mediated syntheses of hydantoins and a bicyclohydantoin under solvent-free conditions”, Tetrahedron Lett. 52, 6148-6151, 2011. |
| [22] | L.E. Seijas, G. E. Delgado, A.J. Mora, A. Bahsas, J. Uzcátegui, “Síntesis y caracterización de los derivados N-carbamoilo e hidantoina de L-prolina”, Av. Quím. 1, 3-7, 2006. |
| [23] | L.E. Seijas, G. E. Delgado, A.J. Mora, A. Bahsas, A. Briceño, “(2S)-1-carbamoylpyrrolidine-2-carboxylic acid”, Acta Cryst. C63, o303-o305, 2007. |
| [24] | G.E. Delgado, A.J. Mora, J. Uzcátegui, A. Bahsas, A. Briceño, “(S)-5-benzylimidazolidine-2,4-dione monohydrate”, Acta Cryst. C63, 448-450, 2007. |
| [25] | L.E. Seijas, A.J. Mora, G.E. Delgado, M. Brunelli, A.N. Fitch, “Study of the conversion of N-carbamoyl-L-proline to hydantoin-L-proline using powder synchrotron X-ray diffraction”, Powder Diffr. 25, 342-348, 2010. |
| [26] | G.E. Delgado, L.E Seijas, A.J. Mora, T. Gonzalez, A. Briceño, “Synthesis, crystal structure and hydrogen-bonding patterns in (RS)-1-carbamoyl pyrrolidine-2-carboxylic acid”, J. Chem. Cryst. 42, 388-393, 2012. |
| [27] | Rigaku, CrystalClear. Rigaku Corporation, Tokyo, Japan, 2002. |
| [28] | Rigaku/MSC, CrystalStructure. Rigaku/MSC, Texas, USA, 2004. |
| [29] | G.M. Sheldrick, “A short history of SHELX”, Acta Cryst. A64, 112-122, 2008. |
| [30] | G. Bergerhoff, M. Berndt, K. Brandenburg, “Evaluation of Crystallographic Data with the Program DIAMOND”, J. Res. Natl. Inst. Stand. Technol. 101, 221-225, 1996. |
| [31] | F. Yu, C.H. Schwalbe, D. Watkin, “Hydantoin and hydrogen-bonding patterns in hydantoin derivatives,” Acta Cryst. C60, 714-717, 2004. |
| [32] | F.H. Allen, “The cambridge structral database: a quarter of a million crystal structures and rising”, Acta Cryst. B58, 380-388, 2002. |
| [33] | E. Arte, B. Tinant, J. Declercq, G. Germain, M. van Meerssche, “Structure of 2 proline hydantoin derivatives l-proline hydantoin and d-allohydroxyproline hydantoin”, Bull. Soc. Chim. Belg. 89, 379-384, 1980. |
| [34] | J.F. Griffin, W. Duax, M. Weeks, “Atlas of Steroid Structure”, New York: Plenum Publishing Corporation, 1984. |
| [35] | M.C. Etter, “Encoding and decoding hydrogen-bond patterns of organic-compounds” Acc. Chem. Res. 23, 120-126, 1990. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML