-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2012; 2(4): 137-140
doi: 10.5923/j.ijmc.20120204.04
Synthesis of 1,3,5-Tris(2-thienyl) Benzene Using CuCl2 as Catalyst, a Compound Precursor of Nano–size Cage-like Structures
Sohrab Abdollahi 1, Fatemeh Mostaghni 1, Nosratallah Mahmoodi 2
1Department of Chemistry, Payame Noor University, I. R. of Iran, P.O. Box 19395-3697, Tehran, Iran
2Department of Chemistry, Gilan University, I. R. of Iran
Correspondence to: Fatemeh Mostaghni , Department of Chemistry, Payame Noor University, I. R. of Iran, P.O. Box 19395-3697, Tehran, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In the previous investigations, the synthesis of 1,3,5-triarylbenzene with different catalysts is reported. In this work, 2-acetyl thiophene and anhydrous copper(II) chloride as catalyst are used to make 1,3,5-tris(2-thienyl)benzene. Copper(II) chloride is a very suitable catalyst and comparing to the other ones is cheaper, abundant and very facile to use in this trimerization synthesis. In fact, CuCl2 acts as a good Lewis acid, because Cu2+ has empty p and d orbitals. On the other hand, this catalyst is a good electron transfer oxidative reagent, therefore very useful for self-condensation of ketones. This method seems to be general for synthesis of other 1,3,5-derivatives of benzene using various ketone derivatives. This trigonal molecule, may be converted to a flexible clathrate or nano cage molecule , which is highly promising for the separation and chemical transformation. In addition, this trigonal molecule which is a good donor, can make complexes with the trigonal acceptors such as 1,3,5-trinitrobenzene.
Keywords: Catalyst, CuCl2, Polythiophenes, Conducting Polymers, Acetylthiophene, Trimerization
Cite this paper: Sohrab Abdollahi , Fatemeh Mostaghni , Nosratallah Mahmoodi , "Synthesis of 1,3,5-Tris(2-thienyl) Benzene Using CuCl2 as Catalyst, a Compound Precursor of Nano–size Cage-like Structures", International Journal of Materials and Chemistry, Vol. 2 No. 4, 2012, pp. 137-140. doi: 10.5923/j.ijmc.20120204.04.
Article Outline
1. Introduction
- In the last decades, due to their molecular properties, conducting polymers have attracted considerable attention regarding modern technologies[1,2,3]. Most of the synthetic researches, in the recent years, have focused on the development of these kinds of material regarding both physical and chemical stabilities. Among polyheterocycle conducting polymers, polythiophenes, are studied more than other systems. Polythiophenes belong to the class of electrically conducting polymers with a non degenerate ground state and a possibility of non linear excitation such as polarons(radical cations or anions) and bipolarons(di cations or di anions)[3,4,5]. In addition, polythiophenes are particularly interesting since sulfur plays a crucial role in partially connecting the π system of consecutive rings and allowing for delocalization of the electronic excitations[5,6]. In this case, polymers can derive their semi- conducting properties by delocalization of π electron along the polymer chain. In fact, π and π* orbitals are the main cause of the electron delocalization. These compounds are used in electrical conductors, electronic devices, electro optics, molecular association with high order and even biological researches[7-11]. These polymers are often produced by oxidative polymerization of thiophene, alkylthiophenes or small oligothiophenes[12]. Recently, it is common to use oligomers with specific structures[13]. During the past several years, it has been clear that the structure can have an essential role in defining the physical properties of conducting polymers. The control of order and arrangement in structure can have considerable effect on electrical properties of the polymer. From trimerization of aryl methyl ketones, at mild condition, with tetrachlorosilane(TCS) catalyst in ethanol, Elmorsy et al. synthesized 1,3,5-triarylbenzene, compound 1, as is shown in Figure 1[14]. In fact the central benzene ring is created by condensation of three ketones via releasing three molecules of water:
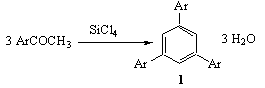 | Figure 1. Condensation Reaction of aryl methyl ketones |
2. Materials and Methods
- Yield refers to isolated pure center cut from column chromatography or to the main bond scratched from preparative TLC plate. Products were characterized by comparison with authentic sample (IR, NMR, TLC and mp) . Melting points determined by Metller FP5 melting point apparatus. IR spectra were obtained on a shimadzu IR-470. The data of NMR were recorded in CDCl3 on Brucker 500 MHZ. TMS was used as an internal reference.Preparation of 1,3,5-tris(2-thienyl)benzene: Anhydrous CuCl2(1 gr, 7.4 mmol) was added to the 2-Acetyl thiophene (6 gr, 4.7 ml). The reaction mixture was refluxed for 3 hr at 130-140℃ on a oil bath. The color of solution was initially yellow orange, but after 15 to 20 min of refluxing, changed to dark purple. During this period the HCl gas was evolved and white CuCl precipitate is formed. Then, it is extracted with ether (3 x 10ml) and washed with water(2 x 10 ml). The combined ether extract was dried(anhydrous Mg SO4) and filtered. The product was obtained by removing the solvent and purified by column chromatography on silicagel and eluted with petroleum ether to obtain the pure product as a colorless crystal(56% isolated yield). Melting point = 160℃ ; IR : 3050, 1430, 1390 and 690 cm-1; 1H-NMR (CDCl3): 7.7 (s, 3H), 6.9-7.8(m, 9H) ; Anal. Calcd For C18H12S3(324.36): C = 66.64; H, 3.69; S, 29.65. Found: C, 66.26; H, 3.63; S, 29.62
3. Results and Discussion
- In this research, the general method of Elmorsy et al. was used to make 1,3,5-tris(2-thienyl)benzene, compound 2 in Figure 2. Therefore, The synthesis of compound 2 is conducted by condensation of three molecules of acetothiophone using copper (II) chloride as catalyst instead of TCS as was used in Elmorsy synthesis . This catalyst is a good electron transfer oxidative reagent, hence very useful for self-condensation of ketones as is shown in Figure 2[16].The structure of the compound 2 shows that this molecule has three sulfur atoms at the ortho position of each thienyl ring which is connected to central benzene ring. The electron pairs of each thienyl ring (two pairs of π electrons and lone pair electron of sulfur) can provide a fine conjugated system with the π electrons of benzene ring[17,18]. As a result, delocalization of the electrons taking place on the whole molecule 2. Therefore the molecule 2 can gain a higher thermodynamic stability due to this delocalization. However, because of the electron pairs on sulfur atoms, the molecule 2 is kinetically reactive and can donate these electrons to the electrophilic(or lewis acid) groups in order to make two or three dimensional polymers[19-21].
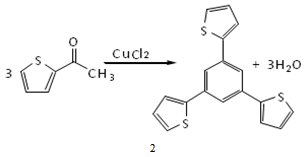 | Figure 2. Self condensation of acetothiophone to make1,2,5-tris(2-thienyl) benzene, using copper(II) chloride as catalyst |
4. Conclusions
- Comparing to the other catalysts for trimerization of methyl ketones, Copper(II) chloride is a very suitable catalyst. It is cheap, abundant and easy to use. We speculate that the method is very convenient and general. It should be applicable to the synthesis of other 1,3,5-derivatives of benzene if different ketone derivatives are used. In this reaction , CuCl2 as a good Lewis acid( Cu2+ has empty p and d orbitals) attacks to the lone electron pairs of oxygen in carbonyl group, making coordinated covalent bond. As a result, this oxygen of carbonyl group can achieve positive charge as is shown in Figure 3. For neutralization of this charge, the hydrogen of α carbon, at high temperature, is easily taken by chloride ion via intermediate 3 to provide enolate 4 . This mechanism may be based on an approximate stoichiometry ratio of 1:1 and structure of yellow color intermediate complex 3 at zero℃ . Even though, there is no any logical evidences for this kind of structure.
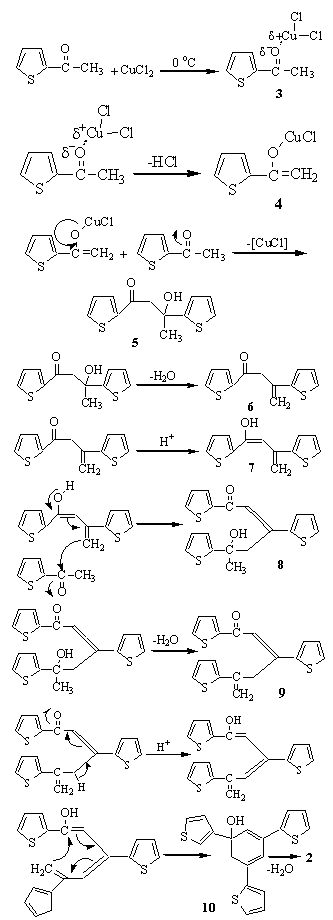 | Figure 3. Trimerization of 2-acetyl thiophene |
 2) CuCl2 →[ CuCl]+ + Cl- 3) 1/2 H2O → H+ + 1/4 O2 + 1e4)[CuCl]+ + 1e → CuCl5) H+ + Cl- → HCl6) 2 CuCl + 2 HCl + 1/2 O2 → 2CuCl2 + H2OFor proving of the last equation, we can refer to the following reasons:1. This is a well known reaction in inorganic chemistry regarding conversion of CuCl to CuCl2.2. Considering the blue color of aqueous solution in this reaction(due to Cu2+), the conversion of CuCl to CuCl2 may be explained by the reaction 6 .Considering the general structure of compound 2, one may predict that the main industrial advantage of this compound is its tendency to produce two dimensional rubber like polymer, compound 3, or three dimensional flexible cage, compound 4, in Figure 4, which is very useful as nano cage in both science and technologies. One area of rapid development in nanotechnology is the ability to synthesize a wide variety of nanostructure compounds. These are, in turn , the result of the availability of a large number of macromolecules of various sizes, shapes, and functionalities. Cage-like nanostructures are particularly interesting because of their applications in storage of molecules for targeted delivery, separation, purification, molecule-specific catalysis and chemical transformations[22]. In addition, if the cage-like nanostructures have functional groups at specific locations, then it is possible that these kind of structures affect the chemistry that requires cooperative effects of various groups as in the case of enzyme catalysis.
2) CuCl2 →[ CuCl]+ + Cl- 3) 1/2 H2O → H+ + 1/4 O2 + 1e4)[CuCl]+ + 1e → CuCl5) H+ + Cl- → HCl6) 2 CuCl + 2 HCl + 1/2 O2 → 2CuCl2 + H2OFor proving of the last equation, we can refer to the following reasons:1. This is a well known reaction in inorganic chemistry regarding conversion of CuCl to CuCl2.2. Considering the blue color of aqueous solution in this reaction(due to Cu2+), the conversion of CuCl to CuCl2 may be explained by the reaction 6 .Considering the general structure of compound 2, one may predict that the main industrial advantage of this compound is its tendency to produce two dimensional rubber like polymer, compound 3, or three dimensional flexible cage, compound 4, in Figure 4, which is very useful as nano cage in both science and technologies. One area of rapid development in nanotechnology is the ability to synthesize a wide variety of nanostructure compounds. These are, in turn , the result of the availability of a large number of macromolecules of various sizes, shapes, and functionalities. Cage-like nanostructures are particularly interesting because of their applications in storage of molecules for targeted delivery, separation, purification, molecule-specific catalysis and chemical transformations[22]. In addition, if the cage-like nanostructures have functional groups at specific locations, then it is possible that these kind of structures affect the chemistry that requires cooperative effects of various groups as in the case of enzyme catalysis. ACKNOWLEDGEMENTS
- This is my pleasure to acknowledge Payame Noor University and Gilan University for their financial supports. I sincerely thank Dr. Saeid Zahmatkesh for his guidance, correction and editing this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML