-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
2012; 2(3): 101-104
doi: 10.5923/j.ijmc.20120203.03
Effect of Potassium Aluminium Sulphate, Kal(SO4)2on the Total Petroleum Hydrocarbon of Diesel Oil
Inimfon A. Udoetok , Nyenime W. Akpanudo , Emaime J. Uwanta , Emmanuel E. Ubuo , Emmanuel J. Ukpong
Chemistry Department, Akwa Ibom State University, IkotAkpaden, MkpatEnin Local Government Area, Akwa Ibom State, Nigeria
Correspondence to: Inimfon A. Udoetok , Chemistry Department, Akwa Ibom State University, IkotAkpaden, MkpatEnin Local Government Area, Akwa Ibom State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Possible effects of the treatment of diesel oil with various concentrations of an inorganic salt, Potassium Aluminium Sulphate (KAl(SO4)2) were studied at room temperature, with a view to assessing alterations in the total petroleum hydrocarbon of diesel oil. Results obtained revealed that various concentrations of the inorganic salt had different effects on the total petroleum hydrocarbon (TPH) of the samples. The sample treated with 55g of the salt had the lowest TPH concentration of 2255.75mg/l while the sample treated with 35g of the salt had the highest TPH concentration of 12505.10mg/l. Two samples had higher TPH concentration than the untreated sample while five samples had lower TPH concentrations. This may be ascribed to the fact that some concentrations of the salt were able to catalytically crack heavier hydrocarbon molecules in the diesel oil to fractions lower than C6 (which may have been gases) thus leading to a decrease in the TPH concentration of those sample, while other concentrations of the salt were able to breakdown heavier hydrocarbon molecules to fractions within the C6 to C40 range thus leading to an increase in the TPH concentrations of the samples. The treatment also led to decrease and increase in the density of the samples.
Keywords: Diesel Oil, Inorganic Salt, Aliphatic Hydrocarbon, TPH, Cracking, Potassium Aluminium Sulphate
Article Outline
1. Introduction
- Petroleum, also known as the black gold isa complex mixture that contains thousands of different organic compounds in three major forms namely crude oil, natural gas and condensates. Crude oil, the liquid form of petroleum is also a complex mixture of hydrocarbons or substituted hydrocarbons varying widely in both physical and chemical properties[1],[2],[3]. It may be characterized in terms of the following fractions namely; saturates, aromatics, resins and asphaltenes as well as heavy metals such as nickel, vanadium and cadmium[4],[5]. It has an average density of 850kg /m3. Saturates include straight or branched chain n-alkanes, and the cycloalkanes with one or more saturated rings, while aromatics include compounds with one or more fused aromatic rings, each of which may have saturated side-chains(alkyl-substituents) attached to them. In contrast to the saturated and aromatic fractions, both resins and asphaltenes consist of non-hydrocarbon polar compounds with trace amounts of nitrogen, sulphur and/or oxygen (NSO) in addition to carbon and hydrogen and often forming complexes with heavy metals[6].When crude oil is distilled, variety of products such asgases, petrol, kerosene, diesel and lubricating oil etc. are obtained at various temperatures[7]. Diesel oil which is one of the distillates is a mixture of hydrocarbons with between 10 to 20 carbon atoms per molecule and distills over a temperature of 250℃ to 400℃[8],[9],[10]. It is used as fuel for heavy duty engines and as raw materials for cracking to obtain motor gasoline. Potash alum (Potassium Aluminium Sulphate) is an inorganic salt with the molecular formula KAl(SO4)2. Potassium Aluminium Sulphate forms a solid, white powder at room temperature. It is a hygroscopic material which when exposed to air, hydrates (absorbs water). Depending on the amount of water molecules present, these hydrates are represented by the chemical formulas KAl(SO4)2 • 12H2O or K2SO4.Al2(SO4)3 • 24H2O. The powder form, made up of crystals, has a melting point of 198.5°F (92.5℃) and can be readily dissolved in water. Additionally, this material has a property known as astringency which is an ability to constrict body tissues, and restrict the flow of blood. There have been many industrial applications of Potassium Aluminium Sulphate. It is an important part of many products created by the pharmaceutical, cosmetic, and food industries because of its astringency property. It is also used in the manufacture of paper, dyes, glue, and explosives. Additionally, it helps in the water purification process, is used to speed up the hardening of concrete and plaster, and acts as a catalyst in various chemical reactions[11],[12],[13]. Reference[14] reported that the treatment of diesel oil with various concentrations of Potash and Alum for a maximum of two days could not make it physically similar to kerosene. He reported that treatment of the sample with 35g of the salts produced the best results. This paper therefore investigates the ability of Potassium Aluminium Sulphate, KAl(SO4)2 to alter the TPH of diesel oil by breaking down high molecular weight hydrocarbon fractions to smaller ones.
2. Experimental
2.1. Sample Collection
- About 1000ml of Diesel oil was collected from Onne port located in Port Harcourt, Rivers state, Nigeriausing a 1000ml glass bottle, while the inorganic salt was obtained from a vendor in Uyo, Akwa Ibom State, Nigeria. On arrival at the laboratory, the diesel oil sample was stored in a refrigerator at 4℃ till commencement of analyses while the Potash alum was stored in a cabinet.
2.2. Sample Preparation
- Potash alum was activated at 80℃ in the oven for about 12 hours. Specified quantities (5g, 10g, 20g, 35g, 55g, 80g and 100g) were introduced into 50mls of the diesel oil in seven preweighed dry and clean 100ml bottles. The mixtures were allowed to react at room temperature for seven days and their weights were taken every morning.
2.3. Oil Extraction and Gas Chromatographic Analyses
- 1g of each of the samples was weighed into well labelled clean and dry vialsand 10mls of pentane was added to them. The samples were stirred using a magnetic stirrer for about 5 minutes before they were allowed to concentrate to 1ml.The extracts were fractionated into aliphatic fractions by adsorption liquid chromatography using a column of alumina and silica gel, while pentane was used as gradient solvent. The extracts were concentrated to 1ml and these were subjected to analyses.The TPH of the samples were determined using a Hewlett Packard 6890 gas chromatograph made by Agilent (USA) with the following operational conditions; flow rate (H2 30ml/min, air 300ml/min and N2 30ml/min), injection temperature (50℃), detector temperature(320℃). For signals, the GC was interfaced to a Hewlett Parker (hp) computer[15].
2.4. Density
- The densities of the samples were measured by taking weights of 50mls of the samples and then the corresponding weights of equal volume of water. The different densities were finally obtained using:Density = weight of sampleWeight of equal volume of water
3. Results and Discussions
- The density of the untreated sample and treated samples are shown in table 1. The result reveals that after treatment of the samples with various mass of the inorganic salt, their densities were lower than that of the untreated sample, with the sample treated with 10g of the salt having the lowest density of 0.769g/cm3. This reduction in density of the treated samples may be due to the breaking down of high molecular weight hydrocarbon fractions within and above the diesel range to smaller hydrocarbons which may have been partly gases. Reference[14] also reported a reduction in the density of diesel from 0.8615g/cm3 to 0.8494g/cm3 when treated with 10g of Potash alum.The results of the gas chromatographic analyses on the treated sample and untreated samples are presented in table 2. The results reveal that the different masses of the salts added to the same volume of diesel oil produced varying effects on the hydrocarbon chain of the diesel. The Total petroleum hydrocarbon of the untreated diesel was 7688.38mg/l (figure 1) whereas after treatment with the inorganic salt, all the samples had different Total petroleum hydrocarbons with some being greater than the untreated sample while some were less than. This shows evidence of alteration of TPH of the diesel oil.
|
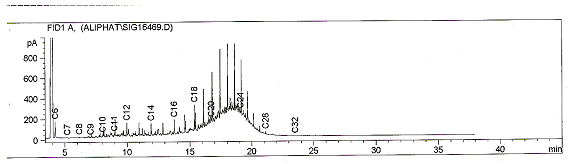 | Figure 1. Chromatogram showing the TPH of diesel |
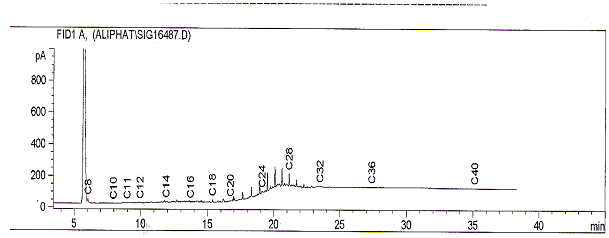 | Figure 2. Chromatogram showing the TPH of sample treated with 55g of the salt |
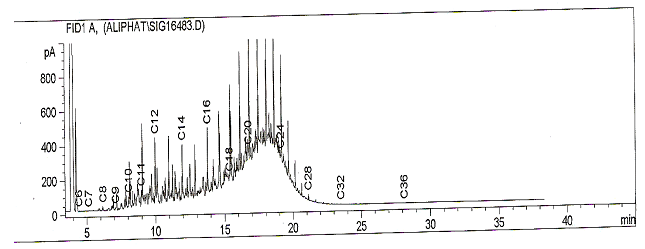 | Figure 3. Chromatogram showing the TPH of sample treated with 35g of the salt |
4. Conclusions
- The result of analyses on diesel oil samples treated with different concentrations of Potassium Aluminium Sulphate revealed that the treatment had variable effects on the density of the samples and on their hydrocarbon chain. These effects included but not limited to the increment and reduction in the TPH concentrations of the samples. While the sample treated with 35g of the salt had the highest TPH value after treatment, the sample treated with 55g of the salt had the lowest. On the whole, it may be concluded that this research has revealed that Potash alum (Potassium Aluminium Sulphate, KAl(SO4)2 has the ability of altering the TPH of diesel oil under suitable conditions by breaking down higher molecular weight hydrocarbon fractions within and above the diesel range to smaller ones.Therefore, there is need for further research to be carried out so as to elucidate the conditions with which diesel oil may be treated with Potassium Aluminium Sulphate to make it similar to kerosene.
ACKNOWLEDGEMENTS
- The authors are grateful to the management of Technology Partners International Nigeria Limited Port Harcourt for facilitating this research.
References
| [1] | Udoetok, I. A. Associated Petroleum Hydrocarbons and Heavy Metals of an Oil Spillage site in Niger-Delta, Nigeria. Global Journal of Pure and Applied sciences, 17, (3), pp 261-265. 2011. |
| [2] | Atlas, R. M., Microbial degradation of petroleum hydrocarbons: An environmental perspective. Microbiol. Rev. 45, 180 -209.1981. |
| [3] | Leahy, J. G., and Colwell, R. R. Microbial degradation of petroleum in the environment. Microbial Reviews, 53 (3): 305 – 315.1990. |
| [4] | "Petroleum". Concise Oxford English Dictionary. |
| [5] | "Petroleum." en.wikipedia.org. 2011.http://en.wikipedia.org/wiki/Petroleum |
| [6] | Udoetok, I. A. Composition and Distribution of Petroleum Hydrocarbons of Idu-Ekpeye Oil Spillage site in Niger-Delta, Nigeria. M.Sc. Thesis, University of Port Harcourt, Nigeria. 2005. |
| [7] | Ababio, O. Y. New School Certificate Chemistry. pp. 83, 84 and 113. Academy Press, Lagos, Nigeria. 1993. |
| [8] | Dyroff, G. V. Manual on significance of test for petroleum products, Chapter 5, 6th ed. Wiley and sons, New York, NY, USA.1993. |
| [9] | http://en.wikipedia.org/wiki/Diesel_fuel#Petroleum_diesel |
| [10] | Chris Collins “Implementing Phytoremediation of Petroleum Hydrocarbons, Methods in Biotechnology 23:99-108. 2007. |
| [11] | http://science.jrank.org/pages/5422/Potassium-Aluminum-Sulfate.html#ixzz1ibE3EqlR. |
| [12] | Bottomley, L. and Bottomley, L.A. School of Chemistry &Bichemistry, Georgia Institute of Technology, Chemistry 1310: Laboratory Manual. Plymouth, MI: Hayden-McNeil Publishing. 2010. |
| [13] | http://simple.wikipedia.org/wiki/Potassium_aluminium_sulfate |
| [14] | Jimoh, A. The use of diesel oil treated with inorganic salt: an alternative to kerosene. AU J. T. 8 (1): 27 – 34. 2004. |
| [15] | Osuji, L. C., Udoetok, I. A. and Ogali, R. E. Attenuation of Petroleum Hydrocarbons by weathering: A case study. Chem. &Biodiv. 3: 422 – 433. 2006. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML