-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2012; 2(2): 79-85
doi: 10.5923/j.ijmc.20120202.07
Thermal Behaviour of Chemically Synthesized Polyanilines/Polystyrene Sulphonic Acid Composites
Gupta Neetika1, Kumar D.2, Tomar S. K.3
1Department of Chemistry, Meerut International Institute of Technology, Meerut, 250002, India
2Department of Chemistry, Delhi Technological University, Delhi, India
3Institute of Engineering & Technology, JK Lakshmipat University; Jaipur, India
Correspondence to: Gupta Neetika, Department of Chemistry, Meerut International Institute of Technology, Meerut, 250002, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Polyanilines are synthesized by free radical chemical oxidative polymerization of aniline & o-toluidine using ammonium persulphate as an oxidant in protonic acid medium. These polymers have been introduced into polystyrene sulphonic acid (PSSA) in 2:1, 1:1, 1:2 compositions for the preparation of composite materials. The composite samples so obtained are thermally characterized by thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), X-ray diffraction, and FTIR spectra. The incorporation of polymer in PSSA has been endorsed by FTIR analysis. TGA data reveals that the (Polyaniline/Polystyrene sulphonic acid) PANI/PSSA (2:1) and (Poly o-toluidine/Polystyrene sulphonic acid) POT/PSSA (2:1) composites show better thermal stability than their 1:1 and 1:2 counterparts. Steric hindrance of –CH3 group present in ortho position and sulphonic acid group of PSSA affects the thermal behaviour of composites. Two-step transitions are clearly reported from the DSC thermograms of polyanilines/PSSA composites and non-reversing chemical cross-linking reaction was further confirmed by X-ray diffraction and DSC thermograms.
Keywords: Polyanilines, Chemical Oxidative Polymerization, Composites, PSSA
Article Outline
1. Introduction
- Conducting polymers have become a popular basic material for advanced applications including plastic batteries, EMI shielding, electrochromic displays and sensors[1-7]. Among conducting polymers, polyaniline (PANI) has been extensively studied for its environmental stability in the conducting form, ease of synthesis, low cost and high conductivity. Nevertheless, a few applications have been reported where conducting polymers like PANI exhibit poor physical and mechanical properties. Numerous methods have been developed to overcome such shortcomings. It has been reported that the composite system by blending PANI with a commodity polymer improves its mechanical properties[8-11]. It has also been proposed that the incorporation of side groups into the main chain[12], grafting of the conducting PANI chain to a non-conductive polymer[13] and electrochemical polymerization of aniline in a polymer matrix[14] improve their processibility and thus broaden their applicability. A large amount of PANI-inorganic composites like PANI/V2O5[15], PANI/TiO2[16-18], PANI/CdS[19], PANI/graphite oxide[20] and PANI/clay[21,22], etc have been synthesized and studied to enhance the overall properties in the past few years. Similarly composites of PANI with polyacrylonitrile, Polyurethane/polymethylmethacrylate (PU/PMMA), Acrylonitrile butadiene styrene (ABS), PVC, Polyvinyledene fluoride/ Nanocrystalline Nickel Composites, Ag-CrO2 Nanocomposite, Polyvinyledene fluoride (PVDF) Based Composites, LiFe1/2Ni1/2VO4 composite were also studied in recent years[23-28].Here, we have developed a simple but interesting method to prepare composites of polyaniline with polystyrene sulphonic acid and poly(o-toluidine) with polystyrene sulphonic acid[29,30] to get better and some new synergistic properties which could not be attained from individual materials. The procedure involves oxidative polymerization of aniline and o-toluidine and synthesis of polystyrene sulphonic acid by the sulphonation of polystyrene separately and subsequently reinforcement of polyaniline and poly(o-toluidine) in polystyrene sulphonic acid. This paper addresses the preparation of composite samples and their thermal behaviour to explore the synergistic properties.
2. Experimental Procedure
2.1. Materials
- Aniline and o-toluidine monomer were double distilled and stored at low temperature prior to use. Polystyrene of grade M110 was procured from Haldia Petrochemicals, India. All other chemicals used were procured from CDH, India. Silver sulphate, ammonium persulphate, HCl and H2SO4 were used as received. Double distilled water was deionized by Millipore used throughout the studies.
2.2. Synthesis of Polyaniline and Poly(o-toluidine)
- The polymerization of freshly distilled aniline has been carried out by free radical chemical oxidative polymerization method by using ammonium persulphate (APS) as an oxidant in non-oxidizing protonic acid like HCl. In a chemical reaction 0.1 M of aniline was mixed to precooled 1 M HCl solution at 0-5℃. An aqueous solution of APS (0.1 M) was added drop wise to reaction mixture with constant stirring for 4-5 h resulted with the stabilization of temperature of reaction mixture till the completion of reaction. The dark green precipitate so obtained was filtered and then washed repeatedly with distilled water till the pH of the filterate became neutral. This precipitate was then dried under dynamic vacuum till constant weight. The polymer was also treated with 1M aqueous ammonia with constant stirring for 3-4 h for converting it into the base/undoped form. The blue black precipitate (undoped form) so obtained was filtered and then washed repeatedly with water to neutral pH. A similar procedure is also employed for the preparation of poly (o-toluidine) undoped form and finally dried under dynamic vacuum.
2.3. Sulphonation of Polystyrene
- Before going to prepare composites of PANI and its derivatives with PSSA, we prepared PSSA from polystyrene through its sulphonation. A mixture of polystyrene granules (3g), silver sulphate (0.034 g) and concentrated H2SO4 (44 mL) was heated at 90℃ for 2 h until it becomes dark brown and highly viscous. This liquid pours into excess of ice-cold distilled water which on constant stirring resulted into a white gummy mass. This precipitate was dissolved in distilled water to make 100 mL solution of PSSA.
2.4. Synthesis of Composites of Polyanilines with PSSA
- For the preparation of composite materials, undoped PANI and POT were mixed with polystyrene sulphonic acid in different compositions, i.e., 1:2, 1:1 and 2:1 by weight. A dark green colour paste was obtained in each case, which was dried in oven at 120℃. These samples of composite materials have been abbreviated as (Polyaniline/Polystyrene sulphonic acid) PANI/PSSA (2:1), PANI/PSSA (1:1), PANI/PSSA (1:2), (Polyo-toluidine/Polystyrene sulphonic acid) POT/PSSA (2:1), POT/PSSA (1:1) and POT/PSSA (1:2) composites.
2.5. Characterization
- The composite material samples along with polyanilines were thermally characterized by TGA, DSC, XRD and FTIR spectra. TGA and DSC were performed on Perkin Elmer by pursuing N2 gas as a carrier at a flow rate of 100 mL/min within a temperature range 50-800℃ for TGA and 50-600℃ for DSC, respectively at a heating rate of 10℃/min. Wide angle X-ray differactometer (model Philips X’pert 1830) using Ni filter and CuKα radiation as a source at 36kV and 15 mA. FTIR spectrum of all composites were taken on FTIR spectrophotometer (Model RX-I Perkin Elmer, UK) in the region of 400-4000 cm-1 at 4 cm-1 resolutions with atleast 32 scans for each sample.
3. Results and Discussion
3.1. Thermogravimeric Analysis
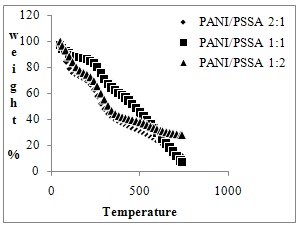 | Figure 1. TGA thermogram of PANI/PSSA composite materials |
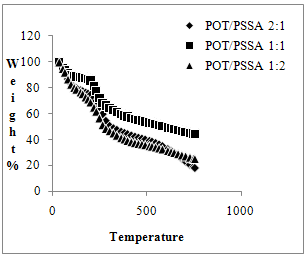 | Figure 2. TGA thermogram of POT/PSSA composite materials |
3.2. Differential Scanning Calorimetry
- DSC thermogram of the polyaniline (EB) form shows an endothermic peak at 50-140℃ and an exothermic peak at 185-350℃. The first peak is most likely attributed to the removal of water and the second peak may be related to the cross-linking reaction [34]. In comparison to polyaniline base form, the composite materials in different compositions show entirely different thermogram as shown in figure3.The thermogram of PANI/PSSA (2:1) composition shows an endothermic peak at 99℃, which may be attributed to the loss of water molecules present in polymer matrix. Another endothermic peak at 272℃ may be assigned to the cross-linking/oxidation of composite backbone, where as the small change above 400℃ onwards may be assigned due to the degradation of the composite backbone. In case of PANI/PSSA (1:1) also, an endothermic peak at 265℃ may be assigned to the cross-linking/oxidation of composite. A transition is observed at about 356℃, which may be attributed due to the decomposition of polymeric system, while in case of PANI/PSSA (1:2) composition, DSC thermogram shows an endothermic peak at about 140℃ due to the evaporation of water molecules trapped inside the composite or bound to the polymer backbone. Other exothermic peak at 298℃ may be assigned due to the cross-linking/oxidation in composite backbone, whereas the change above 360℃ may be assigned due to the degradation of composite. The glass transition was not observable in these composites, because the glass transition is buried in the peak due to the removal of water and it does not exhibit hysteresis. When the polymer powder is heated, cooled and reheated, no exotherm is observed upon heating. However, if the transition is a Tg, it should be observed repeatedly upon heating and cooling. Cross-linking in these polymers is an irreversible chemical reaction and therefore would be observed only upon heating the polymer for the first time.
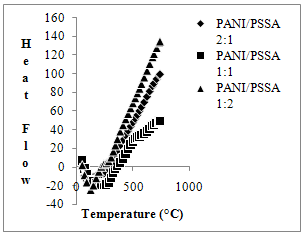 | Figure 3. DSC thermogram of PANI/PSSA composite materials |
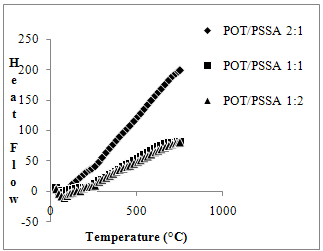 | Figure 4. DSC thermograms of POT/PSSA composite materials |
3.3. X-ray Diffraction
- In PANI/PSSA (2:1) composite, the XRD pattern shows sharp peaks centered on 2θ value of 15, 17 and small peak at 25°, while in case of PANI/PSSA (1:1) composition small peaks at 10, 39 and 45° and in case of PANI/PSSA (1:2) composition small hump at 10, 16, 19, 25 and 390 are observed. The intensity of above peaks decreases abruptly with the increase in PSSA content in the composite material. Thus the peaks appear as weak shoulders in PANI/PSSA (1:1) and PANI/PSSA (1:2) compositions.In POT/PSSA (2:1) composite, the XRD pattern shows sharp peaks centered on 2θ value of 14, 17 20, 39 and 45°, while in case of POT/PSSA (1:1) a small hump at 14, and a broad amorphous peak at 25 are observed. In POT/PSSA (1:2) composite, small hump at 20, 39 and 43 are observed. Thus POT/PSSA (2:1) composite is found more crystalline than POT/PSSA (1:1) and POT/PSSA (1:2) composite, because as PSSA content increases in the composite material, the intermolecular chain spacing and amorphous nature of composites increases due to steric hindrance of methyl group. The amorphous nature in PANI/PSSA (1:2) and POT/PSSA (1:2) composition also attributes the reason that PSSA acts as a plasticizer and it distorts the lattice. Thus instead of entering inside the emplty lattice, it first forms attachment through suitable bonding at the reactive site and simultaneously engulfs the whole polymer in a very regular mannerbecause of its miceller type morphology. This type of morphology destroys the regular arrangement of polymeric backbone and thus leads towards the amorphous character. The Bragg angles and d-spacing obtained from the wide angle X-ray diffractograms of powdered samples of composite materials are presented in Table-1.
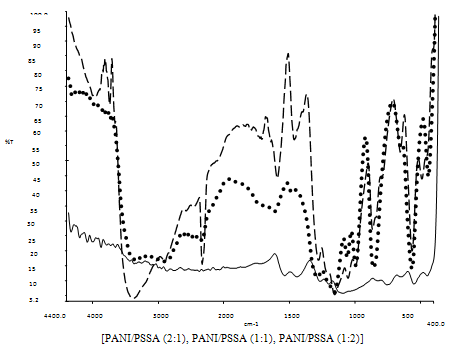 | Figure 5. FTIR spectra of PANI/PSSA composite materials |
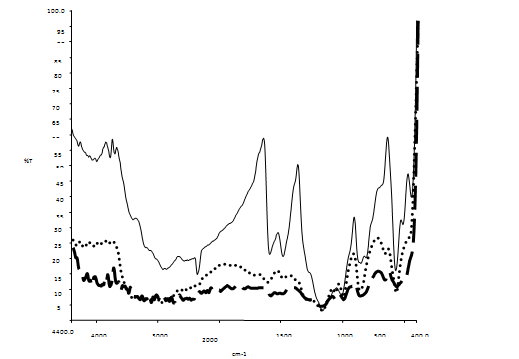 | Figure 6. FTIR spectra of POT/PSSA composite materials[ POT/PSSA (2:1), POT/PSSA (1:1),POT/PSSA (1:2)] |
3.4. FTIR Analysis
- The FTIR spectra of emeraldine base form of aniline are observed in the range of 1650 cm-1 to 1220 cm-1, which arises due to the aromatic ring breathing, -NH- deformation and due to the -C-N- stretching. The bands at 1577 cm-1 and at 1484 cm-1 are the characteristic bands of N- quinoid and benzenoid rings and their presence shows a prominence in the conducting state of the polymer. The absorption band at 1227 cm-1 arises due to C-N benzenoid ring stretching in the polymer. The peak at 818 cm-1 attributes due to the para coupled ring while peak at 886 cm-1 represents the deformed vibrational mode of benzene ring, which is caused due to attachment of specific group present on the ring. Figure5. Shows the FTIR spectra of composite materials prepared by reinforcement of polymer, i.e. polyaniline to PSSA in different compositions such as PANI/PSSA (2:1), PANI/PSSA (1:1) and PANI/PSSA (1:2) compositions. The FTIR studies reveal the formation of composite materials and help to obtain their compositions qualitatively. In case of composites, the out of plane H deformation, i.e. para coupling was observed at ~ 800 cm-1. The band at 1470-1490 cm-1 corresponds to C=C ring vibrations of benzenoid ring. The peak at 1130-1140 corresponds to sulphonic acid group in all these compositions, i.e. due to the symmetric –SO3 stretching. The intensity of this band goes on increasing with the increase in PSSA content. The characteristic bands of both PSSA and PANI confirms the presence of both phases in composite materials, but all these bands shows a systematic shifting that indicates existence of significant interaction between PANI and PSSA in the composite material.The FTIR spectra of poly(o-toluidine) giving peaks at 1591 and 1491 cm-1 are due to the C=C vibration of quinoid and benzenoid rings. The characteristic peak at 1304 cm-1 has been attributed due to C-N in Q-B-Q sequence. The peak at 807 cm-1 attributes to the para coupled phenyl ring and a strong peak at 878 cm-1 appears due to the methyl group attached to phenyl ring. The band at 1155 cm-1 could be attributed to the -CH3 rocking mode. The FTIR spectra of composite materials are shown in figure6. The C=C vibrations of quinoid ring is observed at 1590-1620 cm-1 in all composites. The peak due to C=C vibration of benzenoid ring is observed at 1485-1510 cm-1 in composite materials. The peak due to methyl group attached to the phenyl ring is observed at 850-870 cm-1 . The characteristic peaks observed at 1170-1190 cm-1 are due to symmetric stretching vibrations of -SO3 (polystyrene sulphonic acid). The peak at 3100-3400 cm-1 shows the presence of N-H stretching vibration in all compositions. The intensity of these peaks goes on increasing with the increase in PSSA content.
4. Conclusions
- Polyaniline and poly(o-toluidine) have been reinforced into PSSA in 2:1, 1:1 and 1:2 compositions for the preparation of composite material. TGA-DSC result show that thermal stability of PANI/PSSA (2:1) and POT/PSSA (2:1) is higher than their 1:1 and 1:2 compositions, respectively. In all thermograms, the first peak is observed due to loss of water/moisture and oligomers while the second peak may be due to the crosslinking/oxidation followed by degradation of composite material. From XRD pattern, we infer that crystallinity of PANI/PSSA (2:1) and POT/PSSA (2:1) is higher than their 1:1 and 1:2 conuterparts. PANI/PSSA (2:1) and POT/PSSA (2:1) compositions find an easy way to attack on the suitable sites of polymer, which facilitates the easy movements of polyaniline and poly(o-toluidine) molecular chain, ultimately causes a change and contributes towards the higher crystallinity. The FTIR data demonstrated the interaction between PSSA and polyanilines with the presence of sulphonic acid peaks in all composites. The characteristic bands of PSSA, PANI and POT also confirm the presence of both phases in the materials.
ACKNOWLEDGEMENTS
- A financial support for major research project from the University Grants Commission, New Delhi is acknowledged gratefully. Authors are also thankful to Prof. P.B.Sharma, vice chancellor, Delhi Technological University, Delhi for his kind support and keen interest in the work.
References
| [1] | V. P. Parkhutik, J. M. Martinez-Duart, R. D. Calleja, E. M. Matveeva, J. Electrochem. Soc, 1993, “Deposition of Polyaniline Films onto Porous Silicon Layers, 140, L94-L95. |
| [2] | K.H.Chen, S. M. Yang, 2003” Polyaniline-montmorillonite composite synthesized by electrochemical method” Synth. Met. 135, 151-152. |
| [3] | J. A. Conklin, S. C. Huang, S. M. Huang, T. Wan, R. B. Kaner, 1995, “Thermal properties of polyaniline and poly(aniline-co-o-ethylaniline)” Macromol., 28, 6522-6527. |
| [4] | L. H. Dao, M. Leclerc, J. Guay, J. W. Chevalier,1989, “Synthesis and characterization of substituted poly anilines”Synth. Met. 29, 377-382. |
| [5] | L. Ding, X. Wang, R. V. Gregory, 1999, “Thermal properties of chemically synthesized polyanilines (EB)powder”, Synth. Met., 104, 73-78. |
| [6] | S. H. Goh, H. S. O. Chan, C. H. Ong, 1998, “Miscible blends of conductive polyaniline with tertiary amide polymers” J. Appl. Polym. Sci. 68, 1839-1844. |
| [7] | N. Gupta, 2010,“Investigations on electronically conducting polymers” Ph.D.Thesis, C.C.S.Univ., Meerut, |
| [8] | N. Gupta, D. Kumar, 2009 “Investigation on poly (aniline-co-o-toluidine) / polystyrene sulphonic acid composite”, Ind. J. Eng Mat. Sci, 16, 403-409. |
| [9] | N. Gupta, S. Sharma, I. Mir, D. Kumar, 2006, “Advances in sensors based on conducting polymers”, J. Sci. Ind. Res. 65, 549-557. |
| [10] | K. Gurunathan, D. C. Trivedi, 2000, “Studies on polyaniline and colloidal TiO composites” Mater. Lett.45, 262-268. |
| [11] | K. Gurunathan, D. P. Amalnerkar, D. C. Trivedi, 2003, “Synthesis and characterization of conducting polymer composite (PAn/TiO2) for cathode material in rechargeable battery”Mater. Lett.57, 1642-1648. |
| [12] | T. Jeevananda, Siddaramaiah, 2003, “Synthesis and characterization of polyaniline filled PU/PMMA IPNs” Eur. Polym. J., 39 (3) 569-578. |
| [13] | V. Jousseaume, M. Morsli, A. Bonnet, O. Tesson, S. Lefrant, 1998, “Electrical properties of polyaniline–polystyrene blends above the percolation threshold” J. Appl. Polym. Sci. 67, 1205-1208. |
| [14] | A. Kaynak, J. Unsoworth, R. Clout, A. Mohan, G. Bears, 1994, “A study of microwave transmission, reflection, absorption, and shielding effectiveness of conducting polypyrrole films” J. Appl. Polym. Sci. 54, 269-278. |
| [15] | P. S. Khiew, N. M. Huang, S. Radiman, M. S. Ahmad, 2004, “Synthesis and characterization of conducting polyaniline-coated cadmium sulphide nanocomposites in reverse microemulsion” Mater. Lett., 58, 516-521. |
| [16] | T. Kobayashi, H. Yoneyama, H. Tamura, 1984, “Polyaniline film-coated electrodes as electrochromic display devices” J. Electroanal. Chem. 161, 419-423. |
| [17] | D. Kumar, R. Chandra, 2001, “Thermal behavior of synthetic metals, Polyanilines”, Ind. J. Eng. Mater. Sci., 8 209-214. |
| [18] | D. Kumar, R. C. Sharma, 1998 “Advances in conducting polymers” Eur. Polym. J. 34 1053-1060. |
| [19] | X. Li, G. Wang, D. Lu, 2004, “Surface properties of polyaniline/nano-TiO2 composites” Appl. Surf. Sci, 229, 395-401. |
| [20] | L. F. Malmonge, L. H. Mattoso, 1995, “Electroactive blends of poly(vinylidene fluoride) and polyaniline derivatives” Polymer, 36, 245-249. |
| [21] | M. Mermillod, J. Tanguy, F. Petiot, 1986, “A study of chemically synthesized polypyrrole as electrode material for battery applications” J. Electrochem. Soc.133, 1073-1079. |
| [22] | H. S. Nalwa, 2006, “Handbook of organic conductive molecules and polymer”,Vol. 2, Wiley, New York. |
| [23] | T. F. Otero, J. Rodriguez, E. Angulov, C. Santamarias, 1993, “Artificial muscles from bilayer structures”, Synth. Met., 57, 3713-3717. |
| [24] | W. Pan, S. Yang, G. Li, J. M. Jiang, 2005, “Electrical and structural analysis of conductive polyaniline/polyacrylonitrile composites”, Eur. Polym. J., 41 2127-2133. |
| [25] | M. Panda, V. Srinivas, A. K. Thakur 2008, “On the question of percolation threshold in polyvinylidene fluoride/nanocrystalline nickel composites”, Appl. Phys. Lett., 92 (1) 132905-132907, 2010 “Thermal effects on the percolation behavior of polyvinylidene fluoride/nickel composites”, Appl. Phys. Lett., 117, 3023-3028 |
| [26] | Y. H. Park, H. C. Shin, Y. Lee, Y. Son, D. H. Baik, 1999 “Electrochemical preparation of polypyrrole copolymer films from PSPMS precursor”, Macromol, 32 4615-4618. |
| [27] | Y. H. Park, C.R. Park, 2001, “Preparation of conducting polyacrylonitrile / polyaniline composite films by electrochemical synthesis and their electroactivity”, Synth. Met. 118(1), 187-192. |
| [28] | M. Ram, R. N. P. Choudhary, A.K. Thakur, 2007 “ Preparation and characterization of LiFe1/2Ni 1/2Vo4” Mater. Chem. & Phys., 101 455-463. |
| [29] | N. Shukla, A. Shukla, A. K. Thakur, R. N. P. Choudhary, 2008, “Low temperature ferroelectric behavior of PVDE based composites”, Ind. J. Eng. Mater. Sci., 15 126-128. |
| [30] | G. P. Singh, S. Ram, A. K. Thakur, R. N. P. Choudhary, 2008 “Electrical properties of ferromagnetic AgCro2 particles”, Ind. J. Eng. Mater. Sci., 15 171-175. |
| [31] | S. Wang, Z. Tan, Y. Li, L. Sun, T. Zhang, 2006, “ Synthesis, characterization and thermal analysis of polyaniline/Zro2 composites” Thermochim. Acta, 441, 191-194. |
| [32] | C. G. Wu, Y. C. Liu, S. S. Hsu, 1999 “Assembly of conducting polymer/ metal oxide multilayer in one step” Synth. Met. 102, 1268-1269. |
| [33] | P. Xiao, M. Xiao, P. Liu, K. Gong, 2000, “ Direct synthesis of a polyaniline intercalated graphite oxide nanocomposite” Carbon, 38 626-628. |
| [34] | S. M. Yang, K. H. Chen, 2003, “Synthesis of polyaniline montmorillonite nanocomposites” Synth. Met., 135, 51-52. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML