-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2012; 2(2): 75-78
doi: 10.5923/j.ijmc.20120202.06
Synthesis of Nanowire-Shaped Silver by Polyol Process of Sodium Chloride
Nguyen V. Nghia1, Nguyen N. K. Truong1, Nguyen M. Thong2, Nguyen P. Hung2
1Department of Physics, Quy Nhon University, Quy Nhon City, Binh Dinh Pro., Vietnam
2Department of Chemistry, Quy Nhon University, Quy Nhon City, Binh Dinh Pro., Vietnam
Correspondence to: Nguyen V. Nghia, Department of Physics, Quy Nhon University, Quy Nhon City, Binh Dinh Pro., Vietnam.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Nanowire-shaped silver have been synthesized by the polyol process in ethylene glycol as a reductant, polyvinylpyrrolidone (PVP) as a stabilizer, using a microwave technique. The products were characterized by transmission electron microscopy (TEM). The presence of sodium chloride in the polyol reduction of silver nitrate facilitated the production of silver nanowires. These wires were formed quickly (in approximately 3 minutes microwave heating). It was found that morphologies and sizes of silver nanostructures depended strongly on such experimental parameters as concentrations of PVP, NaCl, AgNO3, and heating time. The chloride ion was necessary to synthesize nanowire-shaped silver, and the sodium chloride likely controlled the rate of silver(I) reduction and initial seed formation.
Keywords: Silver Nanostructures, Chloride Anion, Polyvinylpyrrolidone, Microwave Heating, Polyol Method
Article Outline
1. Introduction
- Among all the nanowire materials, the synthesis of silver nanowires has been and continues to be an area of active research because of their wide applications in catalysts, scanning probes, and various kinds of electronic and photonic nanodevices. Microwave-polyol method is a promising route for rapid preparation of metallic nanomaterials[1]. When microwave was irradiated into the mixture of AgNO3/NaCl/PVP in ethylene glycol solution, anisotropic Ag nanarods and nanowires were produced preferentially. In this work, we examined the dependence of shape, size of silver nanostructures on such experimental parameters as concentrations of PVP, NaCl, AgNO3, and heating time. The changes of shapes and sizes of silver nanostructures were observed by using transmission electron microscope (TEM). Possible formation mechanism of nanorods, nanowires, spherical and cubic nanoparticles under microwave (MW) irradiation in the presence of sodium chloride was discussed.
2. Experimental
- A microwave oven (Sanyo, 2.45Ghz, 800W) was modified by installing a condenser through holes of the ceiling. Polyvinyl pyrrolidone (PVP, Mw=360K Sigma Aldrich, 99.9%) was homogeneously dispersed into ethylene glycol (EG, , 96%) in a 100 mL pyrex becher. Then silver nitrate AgNO3, sodium chloride NaCl were added into the solution and stirred until the AgNO3 and NaCl dissolved completely. The resulted solutions were heated in the microwave oven at 400 W for various concentrations of AgNO3, PVP, NaCl, and heating time. The synthesized silver colloidal solutions were characterized by analytical technique TEM (JEM-1400).
3. Results and Discussion
3.1. Effects of Heating Time
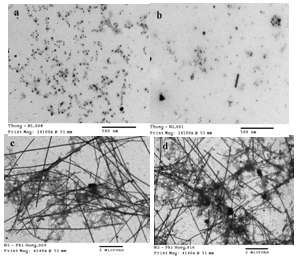 | Figure 1. TEM images of Ag nanostructures synthesized from AgNO3/ NaCl/PVP/EG with the microwave irradiation time 1.5 min (a), 2 min (b), 3.5 min (c), and 5 min (d) |
3.2. Effects of PVP Concentration
- To test the effects of PVP concentration to morphology of Ag nanostructures, nanosized Ag colloids are synthesized at different concentrations of PVP. Figures 2 (a)-(c) show the typical TEM images of nanosized Ag colloids synthesized from AgNO3(60 mM)/NaCl(3 mM)/PVP/EG with PVP concentration of 25 mM, 50 mM, 200 mM, respectively. At the the lowest PVP concentration of 25 mM, aggregation occurs so that large spherical particles are major products. When the PVP concentration increased to 50 mM, the products of nanowires increased considerably. But with increasing PVP concentration too high (200 mM), highly crystalline Ag cubes and triangular bipyramids are preferentially. From the above results, we can conclude that the concentration of PVP has a significant effect on the shape and size of Ag nanostructured.
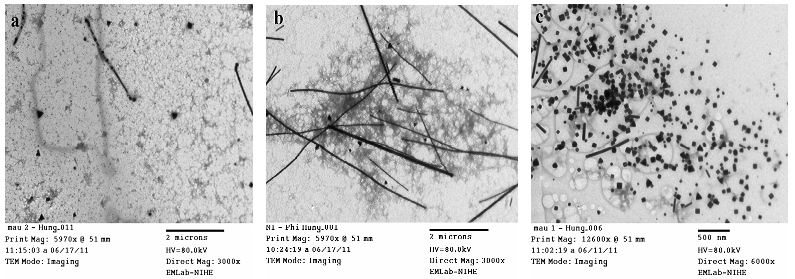 | Figure 2. TEM images of Ag nanostructures synthesized from AgNO3/ NaCl/PVP/EG with PVP concentrations of 25 mM(a), 50 mM(b), 200 mM(c) |
3.3. Effects of AgNO3 Concentration
- Figures 3 (a)-(d) show the typical TEM images of Ag nanostructures synthesized from AgNO3/NaCl(3mM)/PVP(50 mM)/EG at the concentration of AgNO3 of 15 mM, 30 mM, 60 mM, and 90 mM, respectively. From the results of TEM images, we see the shape and size of products depend strongly on the concentration of AgNO3. At the low concentration of AgNO3 15 mM, cubes and triangular bipyramids are dominant products. In contrast, the yields of 1-D (rods and wires) products increase with increasing the concentration of AgNO3. At the highest concentration of AgNO3 90 mM, the yields of 1-D products are more than 80%.
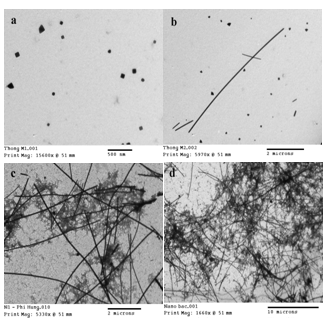 | Figure 3. TEM images of Ag nanostructures are synthesized from AgNO3/NaCl(3 mM)/PVP(50 mM)/EG at the concentrations of AgNO3 of 15 mM (a), 30 mM (b), 60 mM (c), 90 mM (d) |
 | Figure 4. TEM images of Ag nanostructures are synthesized from AgNO3/NaCl/PVP/EG at NaCl concentrations of 0 mM (a), 1 mM (b), 3 mM (c), 10 mM (d) |
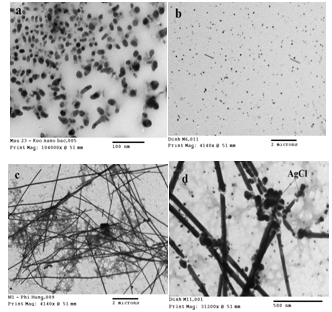
3.4. Effects of NaCl Concentration
- Figures 4 (a)-(d) show the typical TEM images of Ag nanocolloid are synthesized from AgNO3(60 mM)/NaCl/ PVP(50 mM)/EG with NaCl concentrations of 0 mM (a), 1 mM (b), 3 mM (c), and 10 mM (d), respectively. In the absence of NaCl, the main products are the spherical silver nanoparticles (96%) besides there is a small rod-shaped form. At room temperature, the addition of NaCl 1 mM in a solution of AgNO3 leads to a very small amount of soluble AgCl formed due to the small solubility constant of AgCl. Along with the increase of reaction temperature, AgCl is dissolved as Ag+ and Cl-, then Ag+ ions is reduced to Ag0, seed formation and crystal growth take place. Because twinning probability is low, single crystal cubes and single-twin bipyramid are produced preferentially. When NaCl concentration increased, the amount of AgCl is formed more and the standard electrode potential of AgCl/Ag = + 0.2233 (compared to the standard hydrogen electrode) lower electrode of the Ag+/Ag = + 0.799 (compared to the standard hydrogen electrode), most atoms Ag0 not formed from AgCl that Ag+ ions freedom[3]. In general, in the presence of NaCl, the Ag product obtained is a mixture of nanorods, nanowires, triangular bipyramids, spherical and cubic nanoparticles. From the results of TEM images, we see at a concentration of NaCl 3 mM are optimum conditions for synthesis of silver nanowires. However, at higher concentrations of NaCl 10 mM, besides 1D (nanorods and nanowires) products, amorphous AgCl particles also appeared[4].
3.5. Growth mechanisms of Ag nanostructures
- At high temperature dehydrated process occurs:
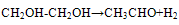 | (1) |
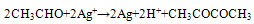 | (2) |
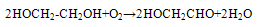 According to these authors, the temperature of reaction was above 150 0C, the reaction formation (3), was producted glycolaldehyde (GA), GA reduces more powerfully than that of acetic aldehyde.Polymer PVP plays a role of developmental control Ag particle size and form Ag products with nanometer size.Growth mechanism of Ag nanostructures under MW- polyol method in the presence of PVP stabilizer and sodium chloride can be described in figure 5[4]. A small amount of sodium chloride is added to act as intermediates to produce the seed in the formation of Ag nano structures. The seeds play an important role in shaping the original shape of Ag nanoparticles with the structure "shell-core": "Ag-seed". While PVP has an important role in taking form the final shape of Ag nanostructures. A low concentrations of seeds and a high concentrations of PVP are favourable for the growth of cubic crystals and the polygonal nanoplates. On other hand, combination of a medium concentration of seeds with low concentrations of PVP are favourable for the growth of nanorods, nanowires and nanosheets[8,9].The presence of trace amounts of chloride ion also acts an important role in the formation of Ag nanowires in ethylene glycol solvent by microwave method. Firstly, it provides electrostatic stabilization for the initial formed silver seeds. Secondly, chloride ion can reduce the concentration of free Ag+ ions in solution through the formation of AgCl nanocrystallites and supply a low release process of Ag+ which facilitates the high-yield formation of the thermodynamically more stable multiple-twined particles (MTPs) required for wire growth. MTPs consist of ten {111}, these {111} faces must remain as active top growth surfaces, while the growth of five side {100} faces must be suppressed by preferential absorption of PVP to {100} faces. Therefore, Ag atoms are preferentially deposited onto{111} surface, leading to the anisotropic growth of silver nanowires[10]. The growth rates to the two opposite <110> directions are nearly the same and the diameter of nanorod is close to the diameter of MTP[9] (figure 6).
According to these authors, the temperature of reaction was above 150 0C, the reaction formation (3), was producted glycolaldehyde (GA), GA reduces more powerfully than that of acetic aldehyde.Polymer PVP plays a role of developmental control Ag particle size and form Ag products with nanometer size.Growth mechanism of Ag nanostructures under MW- polyol method in the presence of PVP stabilizer and sodium chloride can be described in figure 5[4]. A small amount of sodium chloride is added to act as intermediates to produce the seed in the formation of Ag nano structures. The seeds play an important role in shaping the original shape of Ag nanoparticles with the structure "shell-core": "Ag-seed". While PVP has an important role in taking form the final shape of Ag nanostructures. A low concentrations of seeds and a high concentrations of PVP are favourable for the growth of cubic crystals and the polygonal nanoplates. On other hand, combination of a medium concentration of seeds with low concentrations of PVP are favourable for the growth of nanorods, nanowires and nanosheets[8,9].The presence of trace amounts of chloride ion also acts an important role in the formation of Ag nanowires in ethylene glycol solvent by microwave method. Firstly, it provides electrostatic stabilization for the initial formed silver seeds. Secondly, chloride ion can reduce the concentration of free Ag+ ions in solution through the formation of AgCl nanocrystallites and supply a low release process of Ag+ which facilitates the high-yield formation of the thermodynamically more stable multiple-twined particles (MTPs) required for wire growth. MTPs consist of ten {111}, these {111} faces must remain as active top growth surfaces, while the growth of five side {100} faces must be suppressed by preferential absorption of PVP to {100} faces. Therefore, Ag atoms are preferentially deposited onto{111} surface, leading to the anisotropic growth of silver nanowires[10]. The growth rates to the two opposite <110> directions are nearly the same and the diameter of nanorod is close to the diameter of MTP[9] (figure 6).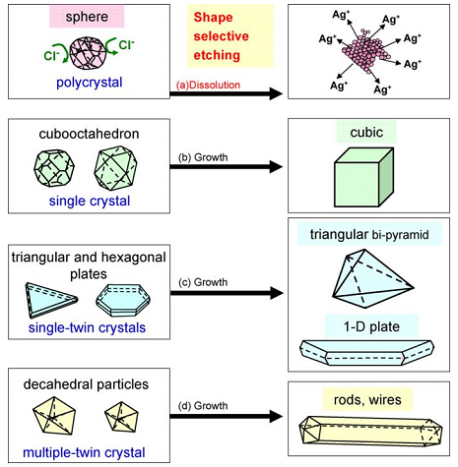 | Figure 5. Growth mechanism of Ag nano-structure products from seed particles |
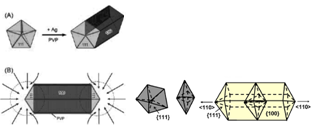 | Figure 6. Growth mechanism of nanorod and nanowire[13] |
4. Conclusions
- In conlusion, we have succeeded in rapid preparation of such anisotropic Ag nanostructures within a few minutes as nanorods, nanowires, and nanocubes by using microwave-polyol method. The shapes and sizes of Ag nanostructures depends strongly on such experimental parameters as concentration of AgNO3, PVP, NaCl and heating time. At low concentration of PVP (50 mM), medium concentration of NaCl (3 mM) and heating time of 3 minutes are favourable for the formation of Ag nanorods and nanowires. The synthesis of Ag nanowires in the presence of NaCl is a new point of this work. The advantages here are simple equipment and process, the much cheaper of NaCl compared to H2PtCl6 in other previous publications.
Acknowledgements
- This work was supported in part by the Grant-in-Aid for Scientific Research from the Ministry of Education and Training, Vietnam (Code B2012.28.47, 2012-2013).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML