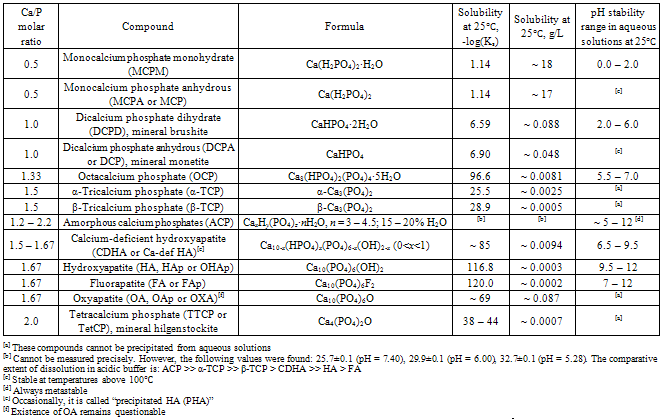
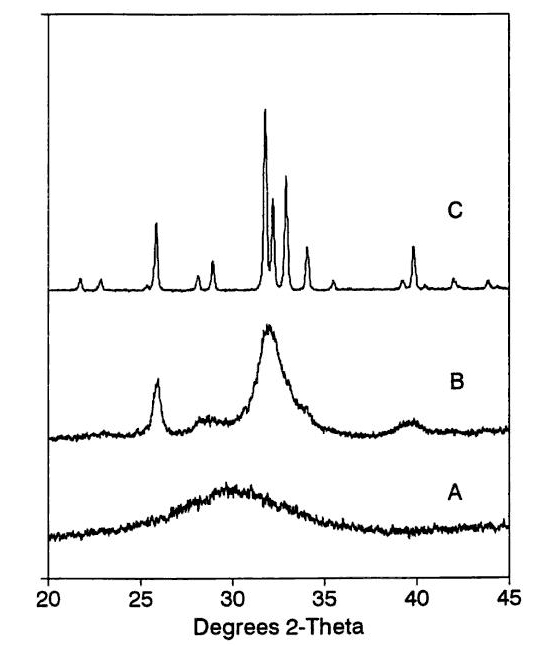
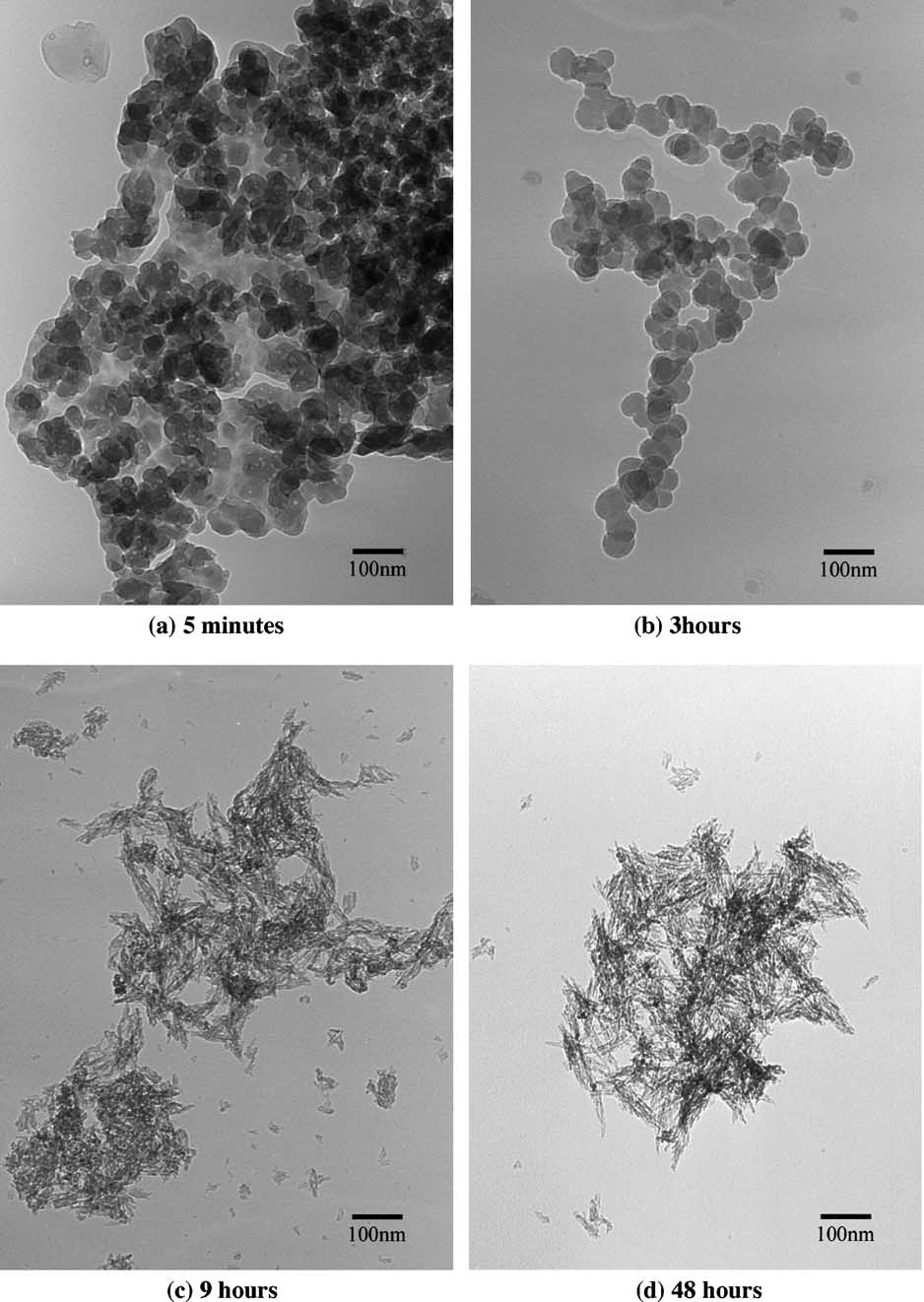
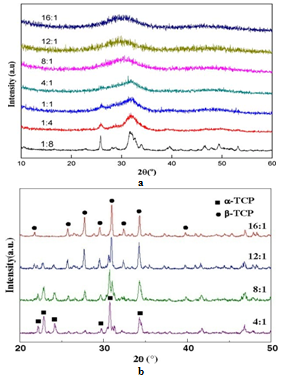
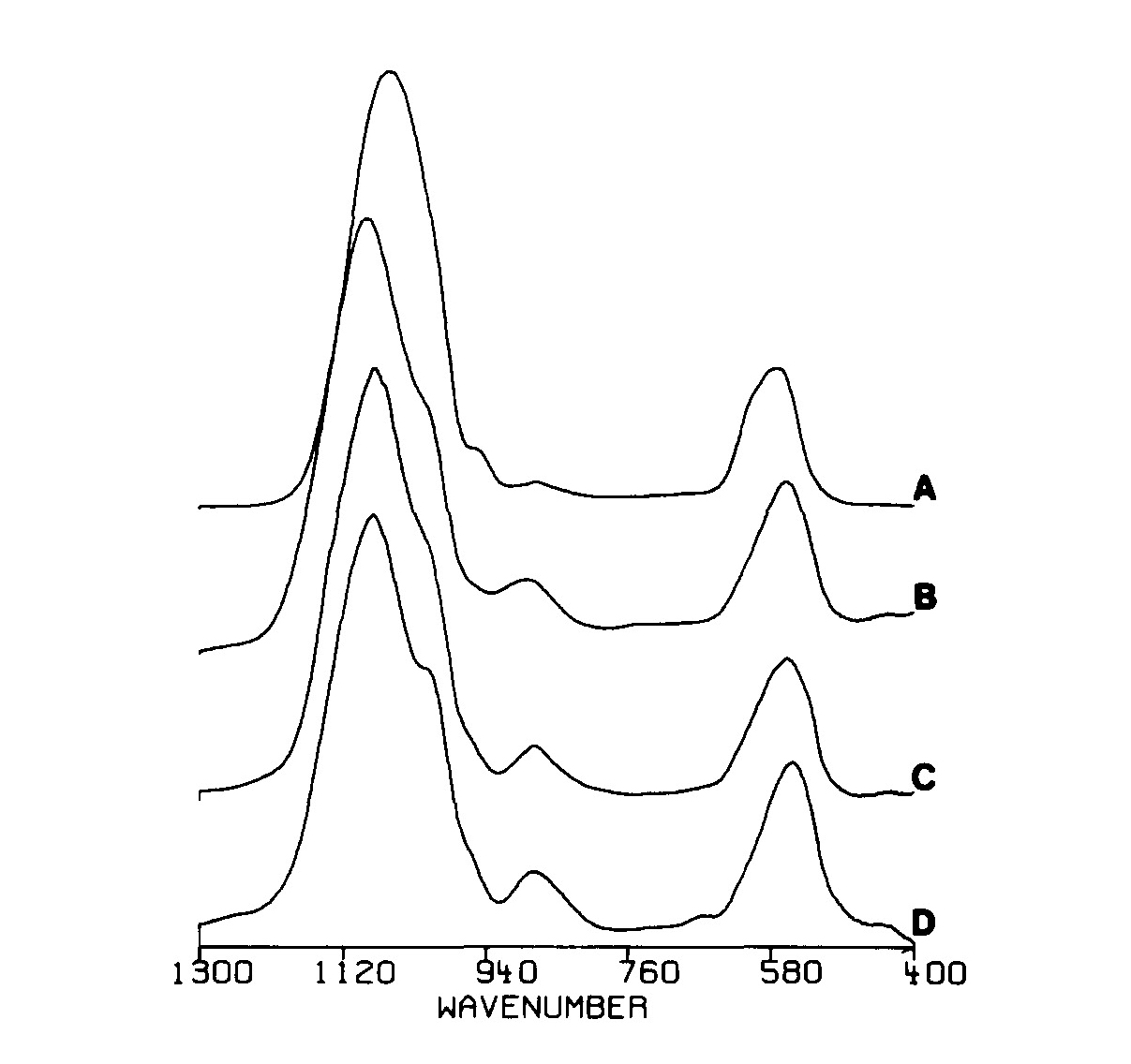
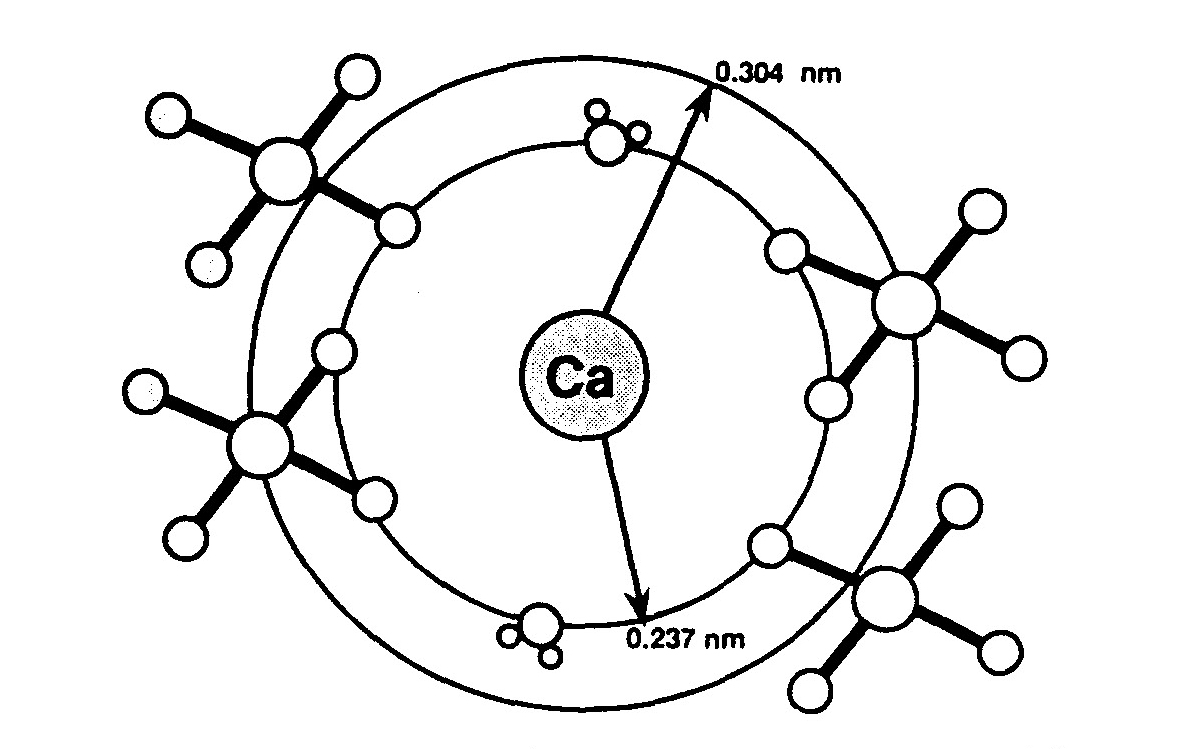
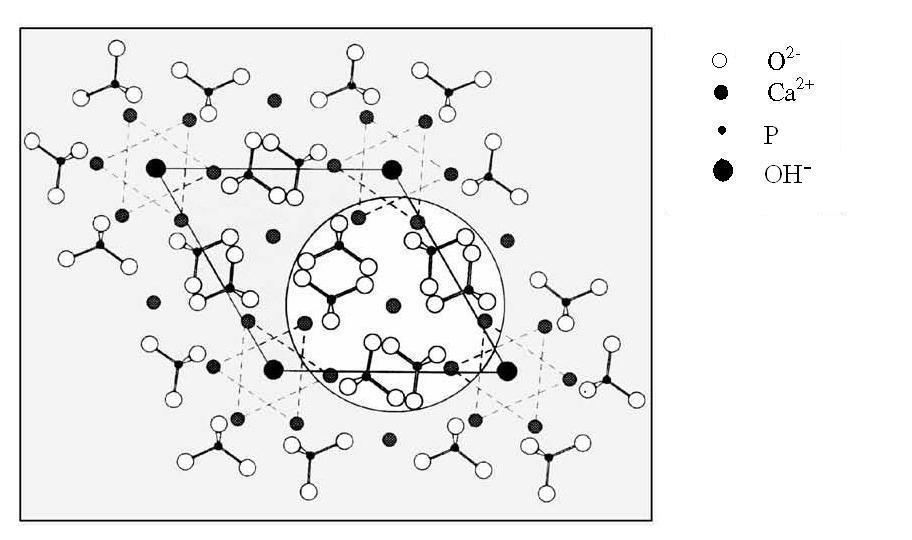
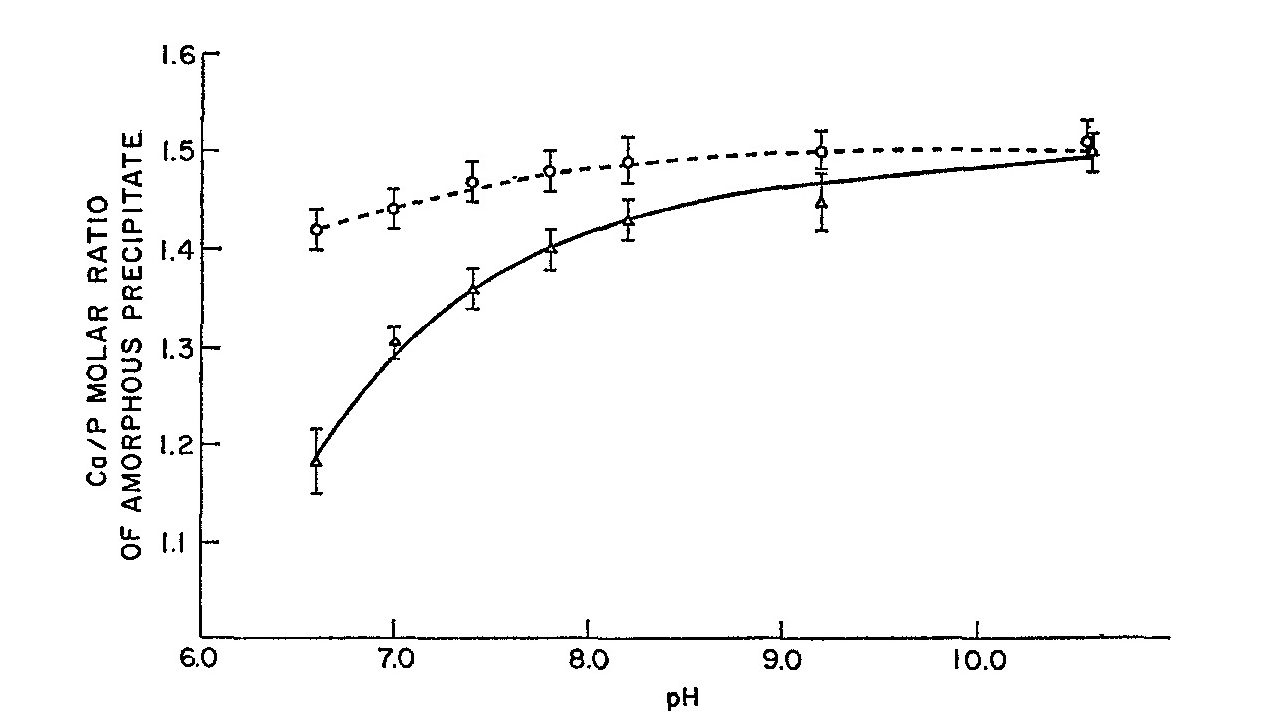
| [1] | Lowenstam, H.A., Weiner, S. On biomineralization. . 1989, 324 pp. |
| [2] | Gower, L.B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 2008, 108, 4551-4627. |
| [3] | Lowenstam, H.A., Weiner, S. Transformation of amorphous calcium phosphate to crystalline dahillite in the radular teeth of chitons. Science 1985, 227, 51-53. |
| [4] | Stricker, S.A., Weiner, S. Amorphous calcium phosphate in the stylets produced by a marine worm (Nemertea). Experientia 1985, 41, 1557-1559. |
| [5] | Mitchell, P.C.H., Parker, S.F., Simkiss, K., Simmons, J., Taylor, M.G. Hydrated sites in biogenic amorphous calcium phosphates: an infrared, Raman, and inelastic neutron scattering study. J. Inorg. Biochem. 1996, 62, 183-197. |
| [6] | Becker, A., Ziegler, A., Epple, M. The mineral phase in the cuticles of two species of Crustacea consists of magnesium calcite, amorphous calcium carbonate, and amorphous calcium phosphate. Dalton Transactions 2005, 10, 1814-1820. |
| [7] | McGann, T.C.A., Buchheim, W., Kearney, R.D., Richardson, T. Composition and ultrastructure of calcium phosphate-citrate complexes in bovine milk systems. Biochim. Biophys. Acta 1983, 760, 415-420. |
| [8] | McGann, T.C.A., Kearney, R.D., Buckheim, W. Amorphous calcium phosphate in casein micelles of bovine milk. Calcif. Tiss. Int. 1983, 35, 821-823. |
| [9] | Brès, E.F., Moebus, G., Kleebe, H.J., Pourroy, G., Werkmann, J., Ehret, G. High resolution electron microscopy study of amorphous calcium phosphate. J. Cryst. Growth 1993, 129, 149-162. |
| [10] | Raeymaekers, L., Agostini, B., Hasselbach, W. The formation of intravesicular calcium phosphate deposits in microsomes of smooth muscle: a comparison with sarcoplasmic reticulum of skeletal muscle. Histochemistry 1981, 70, 139-150. |
| [11] | Termine, J.D., Posner, A.S. Infrared analysis of rat bone: age dependency of amorphous and crystalline mineral fractions. Science 1966, 153, 1523-1525. |
| [12] | Termine, J.D., Wuthier, R.E., Posner, A.S. Amorphous-crystalline mineral changes during endochondral and periosteal bone formation. Proceedings of the Society for Experimental Biology and Medicine 1967, 125, 4-9. |
| [13] | Eanes, E.D., Termine, J.D., Posner, A.S. Amorphous calcium phosphate in skeletal tissues. Clin. Orthop. Relat. Res. 1967, 53, 223-235. |
| [14] | Tannenbaum, P.J., Schraer, H., Posner, A.S. Crystalline changes in avian bone related to the reproductive cycle. II. Percent crystallinity changes. Calcif. Tiss. Int. 1974, 14, 83-86. |
| [15] | Glimcher, M.J., Bonar, L.C., Grynpas, M.D., Landis, W.J., Roufosse, A.H. Recent studies of bone mineral: is the amorphous calcium phosphate theory valid? J. Cryst. Growth 1981, 53, 100-119. |
| [16] | Grynpas, M.D., Bonar, L.C., Glimcher, M.J. On the question of amorphous tricalcium phosphate in bone mineral. Dev. Biochem. 1981, 22, 279-283. |
| [17] | Grynpas, M.D., Bonar, L.C., Glimcher, M.J. Failure to detect an amorphous calcium-phosphate solid phase in bone mineral: a radial distribution function study. Calcif. Tiss. Int. 1984, 36, 291-301. |
| [18] | Aoba, T., Moreno. E. Changes in the nature and composition of enamel mineral during porcine amelogenesis. Calcif. Tiss. Int. 1990, 47, 356-364. |
| [19] | Boskey, A.L. Amorphous calcium phosphate: the contention of bone. J. Dent. Res. 1997, 76, 1433-1436. |
| [20] | Eanes, E.D. Amorphous calcium phosphate. In: Chow, L.C., Eanes, E.D. (Eds): Octacalcium phosphate. Monographs Oral Sci. Vol. 18. Karger, Basel, Switzerland. 2001, pp. 130-147. |
| [21] | Weiner, S., Sagi. I., Addadi, L. Choosing the crystallization path less travelled. Science 2005, 309, 1027-1028. |
| [22] | Weiner, S. Transient precursor strategy in mineral formation of bone. Bone 2006, 39, 431-433. |
| [23] | Suvorova, E.I., Petrenko, P.P., Buffat, P.A. Scanning and transmission electron microscopy for evaluation of order/disorder in bone structure. Scanning 2007, 29, 162-170. |
| [24] | Rey, C., Combes. C., Drouet, C., Glimcher. M.J. Bone mineral: an update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013-1321. |
| [25] | Olszta, M.J., Odom, D.J., Douglas, E.P., Gower, L.B. A new paradigm for biomineral formation: mineralization via an amorphous liquid phase precursor. Connect. Tiss. Res. 2003, 44, 326-334. |
| [26] | Mahamid, J., Sharir. A., Addadi, L., Weiner. S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: indications for an amorphous precursor phase. Proc. Natl. Acad. Sci. USA 2008, 105, 12748-12753. |
| [27] | Mahamid, J., Aichmayer, B., Shimoni, E., Ziblat, R., Li, C., Siegel, S., Paris, O., Fratzl, P., Weiner, S., Addadi, L. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc. Natl. Acad. Sci. USA 2010, 107, 6316-6321. |
| [28] | Tsuji, T., Onuma, K., Yamamoto, A., Iijima, M., Shiba, K. Direct transformation from amorphous to crystalline calcium phosphate facilitated by motif-programmed artificial proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 16866-16870. |
| [29] | Beniash, A., Metzler, R.A., Lam, R.S.K., Gilbert, P.U.P.A. Transient amorphous calcium phosphate in forming enamel. J. Struct. Biol. 2009, 166, 133-143. |
| [30] | Tao, J., Pan, H., Zeng, Y., Xu, R., Tang, R. Roles of amorphous calcium phosphate and biological additives in the assembly of hydroxyapatite nanoparticles. J. Phys. Chem. B 2007, 111, 13410-13418. |
| [31] | Combes, C., Rey, C. Amorphous calcium phosphates: synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362-3378. |
| [32] | Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061-1095. |
| [33] | Dorozhkin, S.V. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399-498. |
| [34] | Holt, C., van Kemenade, M.J.J.M., Harries, J.E., Nelson, L.S. Jr., Bailey, R.T., Hukins, D.W.L., Hasnain, S.S., de Bruyn, P.L. Preparation of amorphous calcium-magnesium phosphates at pH 7 and characterization by X-ray absorption and Fourier transform infrared spectroscopy. J. Cryst. Growth 1988, 92, 239-252. |
| [35] | Bachra, B.N. Precipitation of calcium carbonates and phosphates from metastable solutions. Ann. NY Acad. Sci. 1963, 109, 251-255. |
| [36] | Bachra, B.N., Trautz, O.R., Simon, S.L. Precipitation of calcium carbonates and phosphates under physiological conditions. Arch. Biochem. Biophys. 1963, 103, 124-138. |
| [37] | Bachra, B.N., Trautz, O.R., Simon, S.L. Precipitation of calcium carbonates and phosphates. III. The effect of magnesium and fluoride ions on the spontaneous precipitation of calcium carbonates and phosphates. Arch. Oral Biol. 1965, 10, 731-738. |
| [38] | Greenfield, D.J., Eanes, E.D. Formation chemistry of amorphous calcium phosphates prepared from carbonate containing solutions. Calcif. Tiss. Res. 1972, 9, 152-162. |
| [39] | Olesen, P.T., Steenberg, T., Christensen. E., Bjerrum, N.J. Electrolytic deposition of amorphous and crystalline zinc-calcium phosphates. J. Mater. Sci. 1998, 33, 3059-3063. |
| [40] | Tadic, D., Peters. F., Epple, M. Continuous synthesis of amorphous carbonated apatite. Biomaterials 2002, 23, 2553-2559. |
| [41] | LeGeros. R.Z., Mijares, D., Park, J., Chang, X.F., Khairoun, I., Kijkowska, R., Dias, R., LeGeros, J.P. Amorphous calcium phosphates (ACP): formation and stability. Key Eng. Mater. 2005, 284-286, 7-10. |
| [42] | Aimanova, O.J., LeGeros, R.Z., Sinyayev, V.A. Antimicrobiologic property hydrated amorphous calcium phosphates containing silver. Key Eng. Mater. 2005, 284-286, 439-442. |
| [43] | Skrtic, D., Antonucci, J.M., Eanes, E.D., Brunworth, R.T. Silica- and zirconia-hybridized amorphous calcium phosphate: effect on transformation to hydroxyapatite. J. Biomed. Mater. Res. 2002, 59, 597-604. |
| [44] | Julien, M., Khairoun, I., LeGeros, R.Z., Delplace, S., Pilet, P., Weiss, P., Daculsi, G., Bouler, J.M., Guicheux, J. Physico-chemical–mechanical and in vitro biological properties of calcium phosphate cements with doped amorphous calcium phosphates. Biomaterials 2007, 28, 956-965. |
| [45] | Sinyaev, V.A., LeGeros, R.Z., Levchenko, L.V., Shustikova, E.S., Karzhaubaeva, R.A. State of water in amorphous calcium and calcium-magnesium phosphates. Russ. J. General Chem. 2008, 78, 864-867. |
| [46] | Sinyaev, V.A., Shustikova, E.S., Levchenko, L.V., Karzhaubaeva, R.A., Tokseitova, G.A. Amorphous calcium barium monophosphate and its dehydration in air at room temperature. Russ. J. Appl. Chem. 2008, 81, 1899-1903. |
| [47] | Lee, D., Kumta, P.N. Chemical synthesis and characterization of magnesium substituted amorphous calcium phosphate (MG-ACP). Mater. Sci. Eng. C 2010, 30, 1313-1317. |
| [48] | Taylor, M.G., Simkiss, K., Simmons, J., Wu, L.N.Y., Wuthier, R.E. Structural studies of a phosphatidyl serine-amorphous calcium phosphate complex. Cell. Mol. Life Sci. 1998, 54, 196-202. |
| [49] | Brečević, L., Hlady, V., Füredi-Milhofer, H. Influence of gelatin on the precipitation of amorphous calcium phosphate. Colloids Surf. 1987, 28, 301-313. |
| [50] | Ambrosio, A.M.A., Sahota, J.S., Khan, Y., Laurencin C.T. A novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and characterization. J. Biomed. Mater. Res. 2001, 58, 295-301. |
| [51] | Skrtic, D., Antonucci, J.M., Eanes, E.D., Eichmiller, F.C., Schumacher, G.E. Physicochemical evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J. Biomed. Mater. Res. (Appl. Biomater.) 2000, 53, 381-391. |
| [52] | Skrtic, D., Antonucci, J.M., Eanes, E.D. Effect of the monomer and filler system on the remineralizing potential of bioactive dental composites based on amorphous calcium phosphate. Polym. Adv. Technol. 2001, 12, 369-379. |
| [53] | Skrtic, D., Antonucci, J.M., Eanes, E.D. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J. Res. Natl. Inst. Stands. Technol. 2003, 108, 167-182. |
| [54] | Skrtic, D., Antonucci, J.M., Eanes, E.D., Eidelman, N. Dental composites based on hybrid and surface-modified amorphous calcium phosphates. Biomaterials 2004, 25, 1141-1150. |
| [55] | Skrtic, D., Antonucci, J.M. Matrix resin effects on selected physicochemical properties of amorphous calcium phosphate composites. J. Bioactive Compatible Polym. 2005, 20, 29-49. |
| [56] | Skrtic, D., Antonucci, J.M., Eanes, E.D. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent. Mater. 1996, 12, 295-301. |
| [57] | Skrtic, D., Antonucci, J.M. Dental composites based on amorphous calcium phosphate – resin composition/physicochemical properties study. J. Biomater. Applic. 2007, 21, 375-393. |
| [58] | Skrtic, D., Antonucci, J.M., Liu, D.W. Ethoxylated bisphenol dimethacrylate-based amorphous calcium phosphate composites. Acta Biomater. 2006, 2, 85-94. |
| [59] | Skrtic, D., Hailer A.W., Takagi, S., Antonucci, J.M., Eanes, E.D. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J. Dental Res. 1996, 75, 1679-1686. |
| [60] | Park, M.S., Eanes, E.D., Antonucci, J.M., Skrtic, D. Mechanical properties of bioactive amorphous calcium phosphate/methacrylate composites. Dent. Mater. 1998, 14, 137-141. |
| [61] | Lee, S.Y., Regnault, W.F., Antonucci, J.M., Skrtic, D. Effect of particle size of an amorphous calcium phosphate filler on the mechanical strength and ion release of polymeric composites. J. Biomed. Mater. Res. B (Appl. Biomater.) 2007, 80B, 11-17. |
| [62] | Antonucci, J.M., Liu, D.W., Skrtic, D. Amorphous calcium phosphate based composites: effect of surfactants and poly(ethylene oxide) on filler and composite properties. J. Dispersion Sci. Technol. 2007, 28, 819-824. |
| [63] | Skrtic, D., Lee, S.Y., Antonucci, J.M., Liu, D.W. Amorphous calcium phosphate based polymeric composites: effects of polymer composition and filler’s particle size on composite properties. Key Eng. Mater. 2005, 284-286, 737-740. |
| [64] | O’Donnell, J.N.R., Schumacher, G.E., Antonucci, J.M., Skrtic, D. Adhesion of amorphous calcium phosphate composites bonded to dentin: a study in failure modality. J. Biomed. Mater. Res. B (Appl. Biomater.) 2009, 90B, 238-249. |
| [65] | Antonucci, J.M., O’Donnell, J.N.R., Schumacher, G.E., Skrtic, D. Amorphous calcium phosphate composites and their effect on composite–adhesive–dentin bonding. J. Adhes. Sci. Technol. 2009, 23, 1133-1147. |
| [66] | Reynolds, E.C., Cai, F., Cochrane, N.J., Shen, P., Walker, G.D., Morgan, M.V., Reynolds, C. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J. Dent. Res. 2008, 87, 344-348. |
| [67] | Amjad, Z. Influence of polyelectrolytes on the precipitation of amorphous calcium phosphate. Colloids Surf. 1990, 48, 95-106. |
| [68] | Bar-Yosef, O.P., Govrin-Lippman, R., Garti, N., Füredi-Milhofer, H. The influence of polyelectrolytes on the formation and phase transformation of amorphous calcium phosphate. Cryst. Growth Des. 2004, 4, 177-183. |
| [69] | Cross, K.J., Huq, N.L., Palamara, J.E., Perich, J.W., Reynolds, E.C. Physicochemical characterisation of casein phosphopeptide-amorphous calcium phosphate nanocomplexes. J. Biological Chem. 2005, 280, 15362-15369. |
| [70] | Gutiérrez, M.C., Jobbágy, M., Ferrer, M.L., del Monte, F. Enzymatic synthesis of amorphous calcium phosphate-chitosan nanocomposites and their processing into hierarchical structures. Chem. Mater. 2008, 20, 11-13. |
| [71] | Cushnie, E.K., Khan, Y.M., Laurencin, C.T. Amorphous hydroxyapatite-sintered polymeric scaffolds for bone tissue regeneration: physical characterization studies. J. Biomed. Mater. Res. A 2008, 84A, 54-62. |
| [72] | Ikawa, N., Kimura, T., Oumi, Y., Sano, T. Amino acid containing amorphous calcium phosphates and the rapid transformation into apatite. J. Mater. Chem. 2009, 19, 4906-4913. |
| [73] | Lin, Q., Li, Y., Lan, X., Lu, C., Xu, Z. Preparation of amorphous calcium phosphate/tricalcium silicate composite powders. Adv. Mater. Res. 2009, 79-82, 1643-1646. |
| [74] | Walker, G.D., Cai, F., Shen, P., Adams, G.G., Reynolds, C., Reynolds, E.C. Casein phosphopeptide-amorphous calcium phosphate incorporated into sugar confections inhibits the progression of enamel subsurface lesions in situ. Caries Res. 2010, 44, 33-40. |
| [75] | Antonucci, J.M., Regnault, W.F., Skrtic, D. Polymerization shrinkage and stress development in amorphous calcium phosphate / urethane dimethacrylate polymeric composites. J. Composite Mater. 2010, 44, 355-367. |
| [76] | Sinyaev, V.A., Shustikova, E.S., Levchenko, L.V., Sedunov, A.A. Synthesis and dehydration of amorphous calcium phosphate. Inorg. Mater. 2001, 37, 619-622. |
| [77] | Dion, A., Berno, B., Hall, G., Filiaggi, M.J. The effect of processing on the structural characteristics of vancomycin-loaded amorphous calcium phosphate matrices. Biomaterials 2005, 26, 4486-4494. |
| [78] | Dion, A., Langman, M., Hall, G., Filiaggi, M.J. Vancomycin release behaviour from amorphous calcium polyphosphate matrices intended for osteomyelitis treatment. Biomaterials 2005, 26, 7276-7285. |
| [79] | Lee, B., Kim, M., Choi, S., Lee, Y.K. Amorphous calcium polyphosphate bone regenerative materials based on calcium phosphate glass. Key Eng. Mater. 2009, 396-398, 209-212. |
| [80] | Chen, G., Li, W., Zhao, B., Sun, K. A novel biphasic bone scaffold: β-calcium phosphate and amorphous calcium polyphosphate. J. Am. Ceram. Soc. 2009, 92, 945-948. |
| [81] | Chun, S., Jeong, J.H., Kim, K.M., Kim, S. Biodegradation study of amorphous and crystalline calcium metaphosphate in the SBF and tris-buffer solution. Key Eng. Mater. 2001, 192-195, 131-134. |
| [82] | Cheng, Y.T., Johnson, W.L. Disordered materials: a survey of amorphous solids. Science 1987, 235, 997-1002. |
| [83] | http://en.wikipedia.org/wiki/Amorphous (accessed in October 2011). |
| [84] | Sheng, H.W., Luo, W.K., Alamgir, F.M., Bai, J.M., Ma, E. Atomic packing and short-to-medium-range order in metallic glasses. Nature 2006, 439, 419-425. |
| [85] | Lee, C.Y., Stachurski, Z.H., Welberry, T.R. The geometry, topology and structure of amorphous solids. Acta Mater. 2010, 58, 615-625. |
| [86] | Hufnagel, T.C. Amorphous materials: finding order in disorder. Nature Mater. 2004, 3, 666-667. |
| [87] | Elliott, S.R. Physics of amorphous materials. 2nd Ed. Longman, London, UK. 1990, 481 pp. |
| [88] | Elliott, S.R. Medium-range structural order in covalent amorphous solids. Nature 1991, 354, 445-452. |
| [89] | Salmon, P.S. Amorphous materials: order in disorder. Nature Mater. 2002, 1, 87-88. |
| [90] | Krivanek, O.L., Gaskell, P.H., Howie, A. Seeking order in ‘amorphous’ materials. Nature 1976, 362, 454-457. |
| [91] | Mountjoy, G. Order in two-dimensional projections of thin amorphous three-dimensional structures. J. Phys. Cond. Matter 1999, 11, 2319-2336. |
| [92] | Simon, V., Lazǎr, D., Turcu, R.V.F., Mocuta, H., Magyari. K., Prinz, M., Neumann, M., Simon, S. Atomic environment in sol-gel derived nanocrystalline hydroxyapatite. Mater. Sci. Eng. B 2009, 165, 247-251. |
| [93] | Weeber, A.W., Bakker, H. Amorphization by ball milling. A review. Physica B 1988, 153, 93-135. |
| [94] | Maier, G., Zipper, P., Stubičar, M., Schurz, J. Amorphization of different cellulose samples by ball milling. Cellulose Chem. Technol. 2005, 39, 167-177. |
| [95] | Motta, A.T. Amorphization of intermetallic compounds under irradiation – a review. J. Nucl. Mater. 1997, 244, 227-250. |
| [96] | Edmondson, P.D., Riley, D.J., Birtcher, R.C., Donnelly, S.E. Amorphization of crystalline Si due to heavy and light ion irradiation. J. Appl. Phys. 2009, 106, 043505 (8 pages). |
| [97] | Robinson, R.A., Watson, M.L. Crystal-collagen relationships in bone as observed in the electron microscope. III. Crystal and collagen morphology as a function of age. Ann. NY Acad. Sci. 1955, 60, 596-660. |
| [98] | Watson, M.L., Robinson, R.A. Collagen-crystal relationships in bone. II. Electron microscope study of basic calcium phosphate crystals. Am. J. Anat. 1953, 93, 25-59. |
| [99] | Chow, L.C., Takagi, S., Vogel, G.L. Letter to the Editor. J. Dent. Res. 1998, 77, 6. |
| [100] | Eanes, E.D. Letter to the Editor. J. Dent. Res. 1998, 77, 6. |
| [101] | Harper, R,A., Posner, A.S. Measurement of non-crystalline calcium phosphate in bone mineral. Proc. Soc. Exp. Biol. Med. 1966, 122, 137-142. |
| [102] | Termine, J.D., Posner, A.S. Amorphous/crystalline interrelationships in bone mineral. Calcif. Tiss. Res. 1967, 1, 8-23. |
| [103] | Posner, A.S. Crystal chemistry of bone mineral. Physiol. Rev. 1969, 40, 760-792. |
| [104] | Posner, A.S., Betts, F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc. Chem. Res. 1975, 8, 273-281. |
| [105] | Betts, F., Blumenthal, N.C., Posner, A.S., Becker, G.L., Lehninger, A.L. Atomic structure of intracellular amorphous calcium phosphate deposits. Proc. Nat. Acad. Sci. USA 1975, 72, 2088-2090. |
| [106] | Posner, A.S., Betts, F., Blumenthal, N.C. Formation and structure of synthetic and bone hydroxyapatite. Progr. Cryst. Growth Char. 1980, 3, 49-64. |
| [107] | Bonar, L.C., Roufousse, A.H., Sabine, W.K., Grynpass, M.D., Glimcher, M.J. X-ray diffraction studies of the crystallinity of bone mineral in newly synthesized and density fractionated bone. Calcif. Tiss. Int. 1983, 35, 202-209. |
| [108] | Molnar, Z. Development of the parietal bone of young mice I. Crystals of bone mineral in frozen-dried preparations. J. Ultrastruct. Res. 1959, 3, 39-45. |
| [109] | Molnar, Z. Additional observations on bone crystal dimensions. Clin. Orthop. 1960, 17, 38-42. |
| [110] | Thyberg, J. Electron microscopic studies on the initial phases of calcification in guinea pig epiphyseal cartilage. J. Ultrastruct. Res. 1974, 46, 206-218. |
| [111] | Gay, C.V. The ultrastructure of the extracellular phase of bone as observed in frozen thin sections. Calcif. Tiss. Res. 1977, 23, 215-223. |
| [112] | Schraer, H., Gay, C.V. Matrix vesicles in newly synthesizing bone observed after ultracryotomy and ultramicroincineration. Calcif. Tiss. Res. 1977, 23, 185-188. |
| [113] | Nancollas, G.H., Mohan, M.S. The growth of hydroxyapatite crystals. Arch. Oral Biol. 1970, 15, 731-745. |
| [114] | Blumenthal, N.C., Betts, F., Posner, A.S. Formation and structure of Ca-deficient hydroxyapatite. Calcif. Tiss. Int. 1981, 33, 111-117. |
| [115] | Meyer, J.L. Phase transformations in the spontaneous precipitation of calcium phosphate. Croatica Chim. Acta 1983, 56, 753-767. |
| [116] | Harries, J.E., Hukins, D.W.L., Holt, C., Hasnain. S.S. Conversion of amorphous calcium phosphate into hydroxyapatite investigated by EXAFS spectroscopy. J. Cryst. Growth 1987, 84, 563-570. |
| [117] | Lopez-Valero, I., Gomez-Lorente, C., Boistelle, R. Effects of sodium and ammonium ions on occurrence., evolution and crystallinity of calcium phosphates. J. Cryst. Growth 1992, 121, 297-304. |
| [118] | Lazić, S. Microcrystalline hydroxyapatite formation from alkaline solutions. J. Cryst. Growth 1995, 147, 147-154. |
| [119] | Gadaleta, S.J., Paschalis, E.P., Betts, F., Mendelson, R., Boskey, A.L. Fourier transform infrared spectroscopy of the solution-mediated conversion of amorphous calcium phosphate to hydroxyapatite: new correlations between X-ray diffraction and infrared data. Calcif. Tiss. Int. 1996, 58, 9-16. |
| [120] | Tarasevich, B.J., Chusuei, C.C., Allara, D.L. Nucleation and growth of calcium phosphate from physiological solutions onto self-assembled templates by a solution-formed nucleus mechanism. J. Phys. Chem. B 2003, 107, 10367-10377. |
| [121] | Kim, S., Ryu, H.S., Jung, H.S., Hong, K.S. Influence of Ca/P ratios of starting solutions on the crystallization of amorphous calcium phosphate to hydroxyapatite. Metals Mater. Int. 2004, 10, 171-175. |
| [122] | Kim, S., Ryu, H.S., Shin, H., Jung, H.S., Hong, K.S. Direct observation of hydroxyapatite nucleation from amorphous phase in a stoichiometric calcium/phosphate aqueous solution. Chem. Lett. 2004, 33, 1292-1293. |
| [123] | Wang, L., Nancollas, G.H. Calcium orthophosphates: crystallization and dissolution. Chem. Rev. 2008, 108, 4628-4669. |
| [124] | Brečević, LJ., Füredi-Milhofer, H. Precipitation of calcium phosphates from electrolyte solutions – II. The formation and transformation of the precipitates. Calcif. Tiss. Res. 1972, 10, 82-90. |
| [125] | Liu, C., Huang, Y., Shen, W., Cui, J. Kinetics of hydroxyapatite precipitation at pH 10 to 11. Biomaterials 2001, 22, 301-306. |
| [126] | Boskey, A.L., Posner, A.S. Formation of hydroxyapatite at low supersaturation. J. Phys. Chem. 1976, 80, 40-45. |
| [127] | Feenstra, T.P., de Bruyn, P.L. The Ostwald rule of stages in precipitation from highly supersaturated solutions: a model and its application to the formation of the nonstoichiometric amorphous calcium phosphate precursor phase. J. Coll. Interf. Sci. 1981, 84, 66-72. |
| [128] | Eanes, E.D. Amorphous calcium phosphate: thermodynamic and kinetic considerations. In: Amjad, Z. (Ed): Calcium phosphates in biological and industrial systems. Kluwer. MA, USA, 1998, pp. 21-39. |
| [129] | Blumenthal, N.C., Posner, A.S., Holmes, J.M. Effect of preparation conditions on the properties and transformation of amorphous calcium phosphate. Mater. Res. Bull. 1972, 7, 1181-1189. |
| [130] | Boskey, A.L., Posner, A.S. Conversion of amorphous calcium phosphate to microcrystalline hydroxyapatite. A pH-dependent., solution-mediated., solid-solid conversion. J. Phys. Chem. 1973, 77, 2313-2317. |
| [131] | Li, Y., Weng, W. In vitro synthesis and characterization of amorphous calcium phosphates with various Ca/P atomic ratios. J. Mater. Sci. Mater. Med. 2007, 18, 2303-2308. |
| [132] | Zyman, Z.Z., Rokhmistrov, D.V., Glushko, V.I. Structural and compositional features of amorphous calcium phosphate at the early stage of precipitation. J. Mater. Sci. Mater. Med. 2010, 21, 123-130. |
| [133] | Holt, C., van Kemenade, M.J.J.M., Nelson, L.S. Jr., Hukins, D.W.L., Bailey, R.T., Harries, J.E., Hasnain, S.S., de Bruyn, P.L. Amorphous calcium phosphates prepared at pH 6.5 and 6.0. Mater. Res. Bull. 1989, 23, 55-62. |
| [134] | Eanes, E.D., Gillessen, I.H., Posner, A.S. Intermediate states in the precipitation of hydroxyapatite. Nature 1965, 208, 365-367. |
| [135] | Urch, H., Vallet-Regi, M., Ruiz, L., Gonzalez-Calbet, J.M., Epple M. Calcium phosphate nanoparticles with adjustable dispersability and crystallinity. J. Mater. Chem. 2009, 19, 2166-2171. |
| [136] | Kim, S., Ryu, H.S., Shin, H., Jung, H.S., Hong, K.S. In situ observation of hydroxyapatite nanocrystal formation from amorphous calcium phosphate in calcium-rich solutions. Mater. Chem. Phys. 2005, 91, 500-506. |
| [137] | Termine, J.D., Eanes, E.D. Comparative chemistry of amorphous and apatitic calcium phosphate preparations. Calcif. Tiss. Res 1972, 10, 171-197. |
| [138] | Zahidi, E., Lebugle, A., Bonel, G. Sur une nouvelle classe de materiaux pour protheses osseuses ou dentaires. [A new class of materials for bone or dental prostheses]. Bull. Sot. Chim. Fr. 1985, 4, 523-527. |
| [139] | Lebugle, A., Zahidi, E., Bonel, G. Effect of structure and composition on the thermal decomposition of calcium phosphates (Ca/P = 1.33). React Solids 1986, 2, 151-161. |
| [140] | Layrolle, P., Lebugle, A. Characterization and reactivity of nanosized calcium phosphates prepared in anhydrous ethanol. Chem. Mater. 1994, 6, 1996-2004. |
| [141] | Layrolle, P., Ito, A., Tateishi, T. Sol-gel synthesis of amorphous calcium phosphate and sintering into microporous hydroxyapatite bioceramics. J. Am. Ceram. Soc. 1998, 81, 1421-1428. |
| [142] | Rodrigues, A., Lebugle, A. Behavior in wet atmosphere of an amorphous calcium phosphate with an atomic Ca/P ratio of 1.33. J. Solid State Chem. 1999, 148, 308-315. |
| [143] | Ohta, M., Honma, T., Umesaki, M., Nakahira, A. Synthesis and evaluation of amorphous calcium phosphate (ACP) by various synthesis methods. Key Eng. Mater. 2006, 309-311, 175-178. |
| [144] | Li, Y.B., Weng, W.J., Cheng, K., Du. P.Y., Shen, G., Han, G.R. Complexes of Ca(II) with polymers as precursors for preparation of amorphous calcium phosphate. Mater. Sci. Technol. 2004, 20, 1075-1078. |
| [145] | Bow, J.S., Liou, S.C., Chen, S.Y. Structural characterization of room-temperature synthesized nano-sized β-tricalcium phosphate. Biomaterials 2004, 25, 3155-3161. |
| [146] | Li, Y.B., Weng, W.J., Cheng, K., Du, P.Y., Shen, G., Wang J., Han G.R. Preparation of amorphous calcium phosphate in the presence of poly(ethylene glycol). J. Mater. Sci. Lett. 2003, 22, 1015-1016. |
| [147] | Wang, R., Weng, W., Deng, X., Cheng, K., Liu, X., Du, P., Shen, G., Han, G. Dissolution behavior of submicron biphasic tricalcium phosphate powders. Key Eng. Mater. 2006, 309-311, 223-226. |
| [148] | Li, Y., Wiliana, T., Tam, K.C. Synthesis of amorphous calcium phosphate using various types of cyclodextrins. Mater. Res. Bull. 2007, 42, 820-827. |
| [149] | Tao, J., Pan, H., Zhai, H., Wang, J., Li, L., Wu, J., Jiang, W., Xu, X., Tang, R. Controls of tricalcium phosphate single-crystal formation from its amorphous precursor by interfacial energy. Cryst. Growth Des. 2009, 9, 3154-3160. |
| [150] | Liu, S., Weng, W., Li, Z., Pan, L., Cheng, K., Song, C., Du, P., Shen, G., Han, G. Effect of PEG amount in amorphous calcium phosphate on its crystallized products. J. Mater. Sci. Mater. Med. 2009, 20, 359-363. |
| [151] | Li, Y., Weng, W., Tam, K.C. Novel highly biodegradable biphasic tricalcium phosphates composed of α-tricalcium phosphate and β-tricalcium phosphate. Acta Biomater. 2007, 3, 251-254. |
| [152] | Li, Y., Li, D., Weng, W. In vitro dissolution behavior of biphasic tricalcium phosphate composite powders composed of α-tricalcium phosphate and β-tricalcium phosphate. Key Eng. Mater. 2008, 368-372, 1206-1208. |
| [153] | Rodrigues, A., Lebugle, A. Influence of ethanol in the precipitation medium on the composition., structure and reactivity of tricalcium phosphate. Colloids Surf. A 1998, 145, 191-204. |
| [154] | Heughebaert, J.C., Montel, G. Conversion of amorphous tricalcium phosphate into apatitic tricalcium phosphate. Calcif. Tiss. Int. 1982, 34, S103-S108. |
| [155] | Yu, T., Ye, J., Wang, Y. Synthesis and property of a novel calcium phosphate cement. J. Biomed. Mater. Res. B (Appl. Biomater.) 2009, 90B, 745-751. |
| [156] | Tofighi, A., Palazzolo, R. Calcium phosphate bone cement preparation using mechano-chemical process. Key Eng. Mater. 2005, 284-286, 101-104. |
| [157] | Gbureck, U., Grolms, O., Barralet, J.E., Grover, L.M., Thull, R. Mechanical activation and cement formation of β-tricalcium phosphate. Biomaterials 2003, 24, 4123-4131. |
| [158] | Gbureck, U., Barralet, J.E., Radu, L., Klinger, H., Thull, R. Amorphous α-tricalcium phosphate: preparation and aqueous setting reaction. J. Am. Ceram. Soc. 2004, 87, 1126-1132. |
| [159] | Gbureck, U., Hofmann, M.P., Barralet, J.E. Thermal performance of mechanically activated tetracalcium phosphate. J. Am. Ceram. Soc. 2005, 88, 1327-1330. |
| [160] | Vaidya, S.N., Karunakaran, C., Pande, B.M., Gupta, N.M., Iyer, R.K., Karweer, S.B. Pressure-induced crystalline to amorphous transition in hydroxylapatite. J. Mater. Sci. 1997, 32, 3213-3217. |
| [161] | Vaidya, S.N., Sugandhi, V. Pressure induced amorphization in calcium phosphates. J. Mater. Sci. 1999, 34, 3769-3778. |
| [162] | Lemons, J.E. Hydroxyapatite coatings. Clin. Orthop. 1988, 235, 220-223. |
| [163] | Zyman, Z.Z., Weng, J., Liu, X., Zhang, X., Ma, Z. Amorphous phase and morphological structure of hydroxyapatite plasma coatings. Biomaterials 1993, 14, 225-228. |
| [164] | Weng, J., Liu, X., Zhang, X., Ma, Z., Ji, X., Zyman, Z.Z. Further studies on the plasma-sprayed amorphous phase in hydroxyapatite coatings and its deamorphization. Biomaterials 1993, 14, 578-582. |
| [165] | Tong, W., Chen, J., Zhang, X. Amorphorization and recrystallization during plasma spraying of hydroxyapatite. Biomaterials 1995, 16, 829-832. |
| [166] | Weng, J., Liu, X.G., Li, X.D., Zhang, X.D. Intrinsic factors of apatite influencing its amorphization during plasma-spray coating. Biomaterials 1995, 16, 39-44. |
| [167] | Gross, K.A., Berndt, C.C., Herman, H. Amorphous phase formation in plasma-sprayed hydroxyapatite coatings. J. Biomed. Mater. Res. 1998, 39, 407-414. |
| [168] | Feng, C.F., Khor, K.A., Kweh, S.W.K., Cheang, P. Thermally induced crystallization of amorphous calcium phosphate in plasma-spheroidized hydroxyapatite powders. Mater. Lett. 2000, 46, 229-233. |
| [169] | 1Tong, W., Li, P. In vitro dissolution of amorphous calcium phosphate (Acp) increased the wear particle generation of plasma-sprayed HA coatings. Key Eng. Mater. 2007, 330-332, 561-564. |
| [170] | Gross, K.A., Phillips, M.R. Identification and mapping of the amorphous phase in plasma-sprayed hydroxyapatite coatings using scanning cathodoluminescence microscopy. J. Mater. Sci. Mater. Med. 1998, 9, 797-802. |
| [171] | Carayon, M.T., Lacout, J.L. Study of the Ca/P atomic ratio of the amorphous phase in plasma-sprayed hydroxyapatite coatings. J. Solid State Chem. 2003, 172, 339-350. |
| [172] | Kumar, R., Cheang, P., Khor, K.A. Phase composition and heat of crystallization of amorphous calcium phosphate in ultra-fine radio frequency suspension plasma sprayed hydroxyapatite powders. Acta Mater. 2004, 52, 1171-1181. |
| [173] | Keller, L., Dollase, W.A. X-ray determination of crystalline hydroxyapatite to amorphous calcium-phosphate ratio in plasma sprayed coatings. J. Biomed. Mater. Res. 2000, 49, 244-249. |
| [174] | Gross, K.A., Gross, V., Berndt, C.C. Thermal analysis of amorphous phases in hydroxyapatite coatings. J. Am. Ceram. Soc. 1998, 81, 106-112. |
| [175] | Döbelin, N., Brunner, T.J., Stark, W.J., Eggimann, M., Fisch, M., Bohner, M. Phase evolution of thermally treated amorphous tricalcium phosphate nanoparticles. Key Eng. Mater. 2009, 396-398, 595-598. |
| [176] | Maciejewski, M., Brunner, T.J., Loher, S,F., Stark, W.J., Baiker, A. Phase transitions in amorphous calcium phosphates with different Ca/P ratios. Thermochim. Acta 2008, 468, 75-80. |
| [177] | Tisserand, R., Rebetez, M., Grivet, M., Bouffard, S., Benyagoub, A., Levesque, F., Carpena, J. Comparative amorphization quantification of two apatitic materials irradiated with heavy ions using XRD and RBS results. Nucl. Instrum. Methods Phys. Res. B 2004, 215, 129-136. |
| [178] | Weikusat, C., Glasmacher, U.A., Schuster, B., Trautmann, C., Miletich, R., Neumann, R. Raman study of apatite amorphised with swift heavy ions under various irradiation conditions. Phys. Chem. Minerals 2011, 38, 293-303. |
| [179] | Eanes, E.D., Posner, A.S. Intermediate phases in the basic solution preparation of alkaline earth phosphates. Calcif. Tiss. Res. 1968, 2, 38-48. |
| [180] | Nylen, M.U., Eanes, E.D., Termine, J.D. Molecular and ultrastructural studies of noncrystalline calcium phosphates. Calcif. Tiss. Res. 1972, 9, 95-108. |
| [181] | Eanes, E.D., Termine, J.D., Nylen, M.U. An electron microscopic study of the formation of amorphous calcium phosphate and its transformation to crystalline apatite. Calcif. Tiss. Res. 1973, 12, 143-158. |
| [182] | Eanes, E.D. Amorphous intermediates in the formation of biological apatites. Physico-chimie et cristallographie des apatites d’intérêt biologique. Coll. Int. CNRS 1975, 230, 295-301. |
| [183] | Barton, S.S., Harrison, B.H. Surface and bulk properties of amorphous calcium phosphate. In: Kerker, M. (Ed.): Colloid and Interface Science. Vol. 3. 50th Proceeding Int’l Conf. Academic Press, New York, USA. 1976. p. 71. |
| [184] | Lundager-Madsen, H.E., Lopez-Valero, I., Lopez-Acevedo, V. The formation product of amorphous tricalcium phosphate at 37°C. J. Cryst. Growth 1986, 75, 429-434. |
| [185] | Roberts, J.E., Heughebaert, M., Heughebaert. J.C., Bonar, L.C., Glimcher, M.J., Griffin, R.G. Solid state 31NMR studies of the conversion of amorphous tricalcium phosphate to apatitic tricalcium phosphate. Calcif. Tiss. Int. 1991, 49, 378-382. |
| [186] | Gbureck, U., Barralet, J.E., Thull, R. Thermodynamic study of formation of amorphous β-tricalcium phosphate for calcium phosphate cements. Key Eng. Mater. 2004, 254-256, 249-252. |
| [187] | Brunner, T.J., Bohner, M., Dora, C., Gerber, C., Stark, W.J. Comparison of amorphous TCP nanoparticles to micron-sized α-TCP as starting materials for calcium phosphate cements. J. Biomed. Mater. Res. B (Appl. Biomater.) 2007, 83B, 400-407. |
| [188] | Brunner, T.J., Grass, R.N., Bohner, M., Stark, W.J. Effect of particle size, crystal phase and crystallinity on the reactivity of tricalcium phosphate cements for bone reconstruction. J. Mater. Chem. 2007, 17, 4072-4078. |
| [189] | Somrani, S., Rey, C., Jemal, M. Thermal evolution of amorphous tricalcium phosphate. J. Mater. Chem. 2003, 13, 888-892. |
| [190] | Somrani, S., Banu, M., Jemal, M., Rey, C. Physico-chemical and thermochemical studies of the hydrolytic conversion of amorphous tricalcium phosphate into apatite. J. Solid State Chem. 2005, 178, 1337-1348. |
| [191] | Mohn, D., Ege, D., Feldman, K., Schneider, O.D., Imfeld, T., Boccaccini, A.R., Stark, W.J. Spherical calcium phosphate nanoparticle fillers allow polymer processing of bone fixation devices with high bioactivity. Polym. Eng. Sci. 2010, 50, 952-960. |
| [192] | Dekker, R.J., de Bruijn, J.D., Stigter, M., Barrere, F., Layrolle, P., van Blitterswijk, C.A. Bone tissue engineering on amorphous carbonated apatite and crystalline octacalcium phosphate-coated titanium discs. Biomaterials 2005, 26, 5231-5239. |
| [193] | Amin, M.S., Randeniya, L.K., Bendavid, A., Martin, P.J., Preston, E.W. Amorphous carbonated apatite formation on diamond-like carbon containing titanium oxide. Diamond and Related Materials 2009, 18, 1139-1144. |
| [194] | Imai, H., Kusunoki, M., Hashimoto, Y., Nishikawa, H., Hontsu, S. Evaluation of biological molecular adsorption on hydroxyapatite and amorphous Ca10(PO4)6(OH)2 thin films using QCM method. IEEJ Trans. EIS 2007, 127, 1839-1842. |
| [195] | Holt, C., Wahlgren, N.M., Drakenberg, T. Ability of a β-casein phosphopeptide to modulate the precipitation of calcium phosphate by forming amorphous dicalcium phosphate nanoclusters. Biochem. J. 1996, 314, 1035-1039. |
| [196] | Eanes, E.D., Posner, A.S. Kinetics and mechanism of conversion of noncrystalline calcium phosphate to crystalline hydroxyapatite. Trans. NY Acad. Sci. 1965, 28, 233-241. |
| [197] | Eanes, E.D. Thermochemical studies on amorphous calcium phosphate. Calcif. Tiss. Res. 1970, 5, 133-145. |
| [198] | Oniki, T., Oyamada, M. Surface structure of amorphous calcium phosphate by ESR of VO2+ adsorbed on it. Calcif. Tiss. Int. 1983, 35, 477-480. |
| [199] | Boulet, M., Marier, J.R. Precipitation of calcium phosphates from solutions at near physiological concentrations. Arch. Biochem. 1961, 98, 157-165. |
| [200] | Newesely, H. Changes in crystal types of low solubility calcium phosphates in the presence of accompanying ions. Arch. Oral Biol. 1961, Spec. Suppl. 6, 174-180. |
| [201] | VuiIleumier, C., Lerch, P. Étude de la structure, par la diffraction des rayons X et la microscopic é1ectronique, de l’hydroxylapatite calcique et des orthophosphates dits tri- et octacalcique. Helv. Chim. Acta 1966, 49, 663-670. |
| [202] | Christoffersen, J., Christoffersen, M.R., Kibalczyc, W., Andersen, F.A. A contribution to the understanding of the formation of calcium phosphates. J. Cryst. Growth 1989, 94, 767-777. |
| [203] | Christoffersen, J., Christoffersen, M.R., Kibalczyc, W. Apparent solubilities of two amorphous calcium phosphates and of octacalcium phosphate in the temperature range 30-. J. Cryst. Growth 1990, 106, 349-354. |
| [204] | Kibalczyc, W., Christoffersen, J., Christoffersen, M.R., Zielenkiewicz, A., Zielenkiewicz, W. The effect of magnesium ions on the precipitation of calcium phosphates. J. Cryst. Growth 1990, 106, 355-366. |
| [205] | Francis, M.D., Webb, N.C. Hydroxyapatite formation from a hydrated calcium monohydrogen phosphate precursor. Calcif. Tiss. Res. 1971, 6, 335-342. |
| [206] | Walton, A.G., Bodin, W.J., Furedi, H., Sehwartz, A. Nucleation of calcium phosphate from solution. Can. J. Chem. 1967, 45, 2695-2701. |
| [207] | Meyer, J.L., Eanes, E.D. A thermodynamic analysis of the amorphous to crystalline calcium phosphate transformation. Calcif. Tiss. Res. 1978, 25, 59-68. |
| [208] | Meyer, J.L. Hydroxyl content of solution-precipitated calcium phosphates. Calcif. Tiss. Int. 1979, 27, 153-160. |
| [209] | Jaeger, C., Maltsev, S., Karrasch, A. Progress of structural elucidation of amorphous calcium phosphate (ACP) and hydroxyapatite (HAp): disorder and surfaces as seen by solid state NMR. Key Eng. Mater. 2006, 309-311, 69-72. |
| [210] | Nelson, L.S. Jr., Holt, C., Harries, J.E., Hukins, D.W.L. Amorphous calcium phosphates of different composition give very similar EXAFS spectra. Physica B 1989, 158, 105-106. |
| [211] | Wuthier, R.E., Rice, G.S., Wallace, J.E.B. Jr., Weaver, R.L., LeGeros, R.Z., Eanes, E.D. In vitro precipitation of calcium phosphate under intracellular conditions: formation of brushite from an amorphous precursor in the absence of ATP. Calcif. Tiss. Int. 1985, 37, 401-410. |
| [212] | Holmes, J.M., Beebe, R.A. Surface areas by gas adsorption on amorphous calcium phosphate and crystalline hydroxyapatite. Calcif. Tiss. Res. 1971, 7, 163-174. |
| [213] | Sedlak, J.M., Beebe, R.A. Temperature programmed dehydration of amorphous calcium phosphate. J. Coll. Interf. Sci. 1974, 47, 483-489. |
| [214] | LeGeros, R.Z., Shirra, W.P., Miravite, M.A., LeGeros, J.P. Amorphous calcium phosphates: synthetic and biological. Physico-chimie et cristallographie des apatites d’intérêt biologique. Coll. Int. CNRS 1975, 230, 105-115. |
| [215] | LeGeros, R.Z. Calcium phosphates in oral biology and medicine. Monographs Oral Sci. Vol. 15. Karger, Basel, Switzerland. 1991, 201 pp. |
| [216] | Boskey, A.L., Posner, A.S. Magnesium stabilization ob amorphous calcium phosphate: a kinetic study. Mater. Res. Bull. 1974, 9, 907-916. |
| [217] | Blumenthal, N.C., Betts, F., Posner, A.S. Stabilization of amorphous calcium phosphate by Mg and ATP. Calcif. Tiss. Res. 1977, 23, 245-250. |
| [218] | Abbona, F., Baronnet, A. A XRD and TEM study on the transformation of amorphous calcium phosphate in the presence of magnesium. J. Cryst. Growth 1996, 165, 98-105. |
| [219] | Fleisch, H., Russell, R.G.G., Bisaz, S., Termine, J.D., Posner, A.S. Influence of pyrophosphate on the transformation of amorphous to crystalline calcium phosphate. Calcif. Tiss. Res. 1968, 2, 49-59. |
| [220] | Termine, J.D., Peckauskas, R.A., Posner, A.S. Calcium phosphate formation in vitro. II. Effects of environment on amorphous-crystalline transformation. Arch. Biochem. Biophys. 1970, 140, 318-325. |
| [221] | Bienenstock, A., Posner, A.S. Calculation of the X-ray intensities from arrays of small crystallites of hydroxyapatite. Arch. Biochem. Biophys. 1968, 124, 604-607. |
| [222] | Tropp, J., Blumenthal, N.C., Waugh, J.S. Phosphorus NMR study of solid amorphous calcium phosphate. J. Am. Chem. Soc. 1983, 105, 22-26. |
| [223] | Fawcett, R.W. A radial distribution function analysis of an amorphous calcium phosphate with calcium to phosphorus molar ratio of 1.42. Calcif. Tiss. Res. 1973, 13, 319-325. |
| [224] | Betts, F., Posner, A.S. An X-ray radial distribution study of amorphous calcium phosphate. Mater. Res. Bull. 1974, 9, 353-360. |
| [225] | Betts, F., Posner, A.S. A structural model for amorphous calcium phosphate. Trans. Am. Crystal Assoc. 1974, 10, 73-84. |
| [226] | Eanes, E.D., Powers, L., Costa, J.L. Extended X-ray absorption fine structure (EXAFS) studies on calcium in crystalline and amorphous solids of biological interest. Cell Calcium 1981, 2, 251-262. |
| [227] | Holt, C., Hukins, D.W.L. Structural analysis of the environment of calcium ions in crystalline and amorphous calcium phosphates by X-ray absorption spectroscopy and a hypothesis concerning the biological function of the casein micelle. Int. Dairy J. 1991, 1, 151-165. |
| [228] | Termine, J.D., Lundy, D.R. Vibrational spectra of some phosphate salts amorphous to X-ray diffraction. Calcif. Tiss. Res. 1974, 15, 55-70. |
| [229] | Onuma, K. Recent research on pseudobiological hydroxyapatite crystal growth and phase transition mechanism. Prog. Cryst. Growth Charact. Mater. 2006, 52, 223-245. |
| [230] | Treboux, G., Layrolle, P., Kanzaki, N., Onuma, K., Ito, A. Symmetry of Posner’s cluster. J. Am. Chem. Soc. 2000, 122, 8323-8324. |
| [231] | Georgalis, Y., Kierzek, A.M., Saenger, W. Cluster formation in aqueous electrolyte solutions observed by dynamic light scattering. J. Phys. Chem. B 2000, 104, 3405-3406. |
| [232] | Treboux, G., Layrolle, P., Kanzaki, N., Onuma, K., Ito, A. Existence of Posner’s cluster in vacuum. J. Phys. Chem. A 2000, 104, 5111-5114. |
| [233] | Kanzaki, N., Treboux, G., Onuma, K., Tsutsumi, S., Ito, A. Calcium phosphate clusters. Biomaterials 2001, 22, 2921-2929. |
| [234] | Boldeskul, I.E., Sukhodub, L.F., Kalinkevich, A.N., Khavryutchenko, V.D. Ab initio modelling of calcium phosphate clusters and their vibrational spectra. Cond. Matter Phys. 2006, 9, 669-679. |
| [235] | Zahn, D. Mechanisms of calcium and phosphate ion association in aqueous solution. Zeitschrift fur Anorganische und Allgemeine Chemie 2004, 630, 1507-1511. |
| [236] | Wang, C.G., Liao, J.W., Gou, B.D., Huang, J., Tang, R.K., Tao, J.H., Zhang, T.L., Wang, K. Crystallization at multiple sites inside particles of amorphous calcium phosphate. Cryst. Growth Des. 2009, 9, 2620-2626. |
| [237] | Kojima, Y., Sakama, K., Toyama, T., Yasue, T., Arai, Y. Dehydration of water molecules in amorphous calcium phosphate. Phosphorus Res. Bull. 1994, 4, 47-52. |
| [238] | Wikholm, N.W., Beebe, R.A., Kittelberger, J.S. Kinetics of the conversion of monetite to calcium pyrophosphate. J. Phys. Chem. 1975, 79, 853-856. |
| [239] | Gross, K.A., Saber-Samandari, S., Heemann, K.S. Evaluation of commercial implants with nanoindentation defines future development needs for hydroxyapatite coatings. J. Biomed. Mater. Res. B (Appl. Biomater.) 2010, 93B, 1-8. |
| [240] | Saber-Samandari, S., Gross, K.A. The use of thermal printing to control the properties of calcium phosphate deposits. Biomaterials 2010, 31, 6386-6393. |
| [241] | Saber-Samandari, S., Gross, K.A. Amorphous calcium phosphate offers improved crack resistance: a design feature from nature? Acta Biomater. 2011, 7, 4235-4241. |
| [242] | Eanes, E.D., Meyer, J.L. The maturation of crystalline calcium phosphates in aqueous suspensions at physiologic pH. Calcif. Tiss. Res. 1977, 23, 259-269. |
| [243] | Meyer, J.L., Weatherall, C.C. Amorphous to crystalline calcium phosphate phase transformation at elevated pH. J. Coll. Interf. Sci. 1982, 89, 257-267. |
| [244] | Ajibola, V.O., Thomas, S.A. Transformation of amorphous calcium phosphate hydroxyapatite in the presence of some ions. Bull. Chem. Soc. Ethiopia 1997, 11, 19-24. |
| [245] | Root, M.J. Inhibition of the amorphous calcium phosphate phase transition reaction by polyphosphates and metal ions. Calcif. Tiss. Int. 1990, 47, 112-116. |
| [246] | Wuthier, R.E., Eanes, E.D. Effect of phospholipids on the transformation of amorphous calcium phosphate to hydroxyapatite in vitro. Calcif. Tiss. Int. 1975, 19, 197-210. |
| [247] | Termine, J.D., Eanes, E.D., Conn, K.M. Phosphoprotein modulation of apatite crystallization. Calcif. Tiss. Int. 1980, 31, 247-251. |
| [248] | Qiu, S.M., Wen, G., Hirakawa, N., Soloway, R.D., Hong, N.K., Crowther, R.S. Glycochenodeoxycholic acid inhibits calcium phosphate precipitation in vitro by preventing the transformation of amorphous calcium phosphate to calcium hydroxyapatite. J. Clin. Investigation 1991, 88, 1265-1271. |
| [249] | Biumenthal, N.C., Betts, F., Posner, A.S. Nucleotide stabilization of amorphous calcium phosphate. Mater. Res. Bull. 1975, 10, 1055-1060. |
| [250] | Termine, J.D., Conn, K.M. Inhibition of apatite formation by phosphorylated metabolites and macromolecules. Calcif. Tiss. Res. 1976, 22, 149-157. |
| [251] | Tung, M.S., Brown, W.E. An intermediate state in hydrolysis of amorphous calcium phosphate. Calcif. Tiss. Int. 1983, 35, 783-790. |
| [252] | Kazanci, M., Fratzl, P., Klaushofer, K., Paschalis, E.P. Complementary information on in vitro conversion of amorphous (precursor) calcium phosphate to hydroxyapatite from Raman microspectroscopy and wide-angle X-ray scattering. Calcif. Tiss. Int. 2006, 79, 354-359. |
| [253] | Pekounov, Y., Petrov, O.E. Bone resembling apatite by amorphous-to-crystalline transition driven self-organisation. J. Mater. Sci. Mater. Med. 2008, 19, 753-759. |
| [254] | Tao, J., Pan, H., Wang, J., Wu, J., Wang, B., Xu, X., Tang, R. Evolution of amorphous calcium phosphate to hydroxyapatite probed by gold nanoparticles. J. Phys. Chem. C 2008, 112, 14929-14933. |
| [255] | Rabadjieva, D., Gergulova, R., Titorenkova, R., Tepavitcharova, S., Dyulgerova, E., Balarew, C., Petrov, O. Biomimetic transformations of amorphous calcium phosphate: kinetic and thermodynamic studies. J. Mater. Sci. Mater. Med. 2010, 21, 2501-2509. |
| [256] | Pan, H., Liu, X.Y., Tang, R., Xu, H.Y. Mystery of the transformation from amorphous calcium phosphate to hydroxyapatite. Chem. Comm. 2010, 46, 7415-7417. |
| [257] | Sugiura, Y., Onuma, K., Kimura, Y., Miura, H., Tsukamoto, K. Morphological evolution of precipitates during transformation of amorphous calcium phosphate into octacalcium phosphate in relation to role of intermediate phase. J. Cryst. Growth 2011, 332, 58-67. |
| [258] | Onuma, K., Ito, A. Cluster growth model for hydroxyapatite. Chem. Mater. 1998, 10, 3346-3351. |
| [259] | Oyane, A., Onuma, K., Kokubo, T., Ito, A. Clustering of calcium phosphate in the system CaCl2–H3PO4–KCl–H2O. J. Phys. Chem. B 1999, 103, 8230-8235. |
| [260] | Yin, X., Stott, M.J. Biological calcium phosphates and Posner’s cluster. J. Chem. Phys. 2003, 118, 3717-3723. |
| [261] | Eanes, E.D. The interaction of supersaturated calcium phosphate solutions with apatitic substrates. Calcif. Tiss. Res. 1976, 20, 75-89. |
| [262] | Eanes, E.D. Crystal growth of mineral phases in skeletal tissues. Progr. Cryst. Growth Character. 1980, 3, 3-15. |
| [263] | Termine, J.D., Eanes, E.D. Calcium phosphate deposition from balanced salt solutions. Calcif. Tiss. Res. 1974, 15, 81-84. |
| [264] | Soares, A.M.V., Arana-Chavez, V.E., Reid, A.R., Katchburian, E. Lanthanum tracer and freeze-fracture studies suggest that compartmentalization of early bone matrix may be related to initial mineralization. J. Anat. 1992, 181, 345-356. |
| [265] | Bonucci, E. Fine structure of early cartilage calcification. J. Ultrastruct. Res. 1967, 20, 33-50. |
| [266] | Anderson, H.C. Vesicles associated with calcification in the matrix of epiphyscal cartilage. J. Cell Biol. 1969, 41, 59-72. |
| [267] | Bernard, G.W., Pease, D.C. An electron microscopic study of initial intramembranous osteogenesis. Am. J. Anat. 1969, 125, 271-290. |
| [268] | Wuthier, R.E. Electrolytes of isolated epiphyseal chondrocytes, matrix vesicles, and extracellular fluid. Calcif. Tiss. Res. 1977, 23, 125-133. |
| [269] | Wuthier, R.E., Gore, S.T. Partition of inorganic ions und phospholipids in isolated cell, membrane and matrix vesicle fractions: evidence for Ca-Pi-acidic phospholipid complexes. Calcif. Tiss. Res. 1977, 24, 163-171. |
| [270] | Eanes, E.D., Hailer, AW., Costa, J.L. Calcium phosphate formation in aqueous suspensions of multilamellar liposomes. Calcif. Tiss. Int. 1984, 36, 421-430. |
| [271] | Sauer, G.R., Zunie, W.B., Durig, J.R., Wuthier, R.E. Fourier-transform Raman-spectroscopy of synthetic and biological calcium phosphates. Calcif. Tiss. Int. 1994, 54, 414-420. |
| [272] | Sauer, G.R., Wuthier, R.E. Fourier-transform infrared characterization of mineral phases formed during induction of mineralization by collagenase-released matrix vesicles in vitro. J. Biol. Chem. 1988, 263, 13718-13724. |
| [273] | Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465-1485. |
| [274] | Dorozhkin, S.V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008, 43, 3028-3057. |
| [275] | Dorozhkin, S.V. Calcium orthophosphate cements and concretes. Materials 2009, 2, 221-291. |
| [276] | Maxian, S.H., Zawadsky, J.P., Dunn, M.G. In vitro evaluation of amorphous calcium phosphate and poorly crysiallized hydroxyapatite coatings on titanium implants. J. Biomed. Mater. Res. 1993, 27, 111-117. |
| [277] | Maxian, S.H., Zawadsky, J.P., Dunn, M.G. Mechanical and histological evaluation of amorphous calcium phosphate and poorly crystallized hydroxyapatite coatings on titanium implants. J. Biomed. Mater. Res. 1993, 27, 717-728. |
| [278] | Garcia, F., Arias, J.L., Mayor, B., Pou, J., Rehman, I., Knowles, J., Best, S.M., León, B., Pérez-Amor, M., Bonfield, W. Effect of heat treatment on pulsed laser deposited amorphous calcium phosphate coatings. J. Biomed. Mater. Res. (Appl. Biomater.) 1998, 43, 69-76. |
| [279] | Heimann, R.B., Wirth, R. Formation and transformation of amorphous calcium phosphates on titanium alloy surfaces during atmospheric plasma spraying and their subsequent in vitro performance. Biomaterials 2006, 27, 823-831. |
| [280] | Liu, D.M., Chou, H.M., Wu, J.D., Tung, M.S. Hydroxyl apatite coating via amorphous calcium phosphate. Mater. Chem. Phys. 1994, 37, 39-44. |
| [281] | Nagano, M., Nakamura, T., Kokubo, T., Tanahashi, M., Ogawa, M. Differences of bone bonding ability and degradation behaviour in vivo between amorphous calcium phosphate and highly crystalline hydroxyapatite coating. Biomaterials 1996, 17, 1771-1777. |
| [282] | Leeuwenburgh, S.C,G., Layrolle, P., Barrère, F., de Bruijn, J., Schoonman, J., van Blitterswijk, C.A., de Groot, K. Osteoclastic resorption of biomimetic calcium phosphate coatings in vitro. J. Biomed. Mater. Res. 2001, 56, 208-215. |
| [283] | Habibovic, P., Barrère, F., van Blitterswijk, C.A., de Groot, K., Layrolle, P. Biomimetic hydroxyapatite coating on metal implants. J. Am. Ceram. Soc. 2002, 85, 517-522. |
| [284] | Rössler, S., Sewing, A., Stolzel, M., Born, R., Scharnweber, D., Dard, M., Worch, H. Electrochemically assisted deposition of thin calcium phosphate coatings at near-physiological pH and temperature. J. Biomed. Mater. Res. A 2003, 64A, 655-663. |
| [285] | Lee, D.D., Tofighi, A., Aiolova, M., Chakravarthy, P., Catalano, A., Majahad, A., Knaack, D. α-BSM®: a biomimetic bone substitute and drug delivery vehicle. Clin. Orthop. Relat. Res. 1999, 367, Suppl., S396-S405. |
| [286] | Tofighi, A., Mounic, S., Chakravarthy, P., Rey, C., Lee, D. Setting reactions involved in injectable cements based on amorphous calcium phosphate. Key Eng. Mater. 2000, 192, 769-772. |
| [287] | Wang, X., Ye, J., Wang, Y., Wu, X., Bai, B. Control of crystallinity of hydrated products in a calcium phosphate bone cement. J. Biomed. Mater. Res. A 2007, 81A, 781-790. |
| [288] | van den Vreken, N.M.F., Pieters, I.Y., Declercq, H.A., Cornelissen, M.J., Verbeeck R.M.H. Characterization of calcium phosphate cements modified by addition of amorphous calcium phosphate. Acta Biomater. 2010, 6, 617-625. |
| [289] | Drouet, C., Largeot, C., Raimbeaux, G., Estournès, C., Dechambre, G., Combes, C., Rey, C. Bioceramics: spark plasma sintering (SPS) of calcium phosphates. Adv. Sci. Technol. 2006, 49, 45-50. |
| [290] | Mazzaoui, S.A., Burrow, M.F., Tyas, M.J., Dashper, S.G., Eakins, D., Reynolds, E.C. Incorporation of casein phosphopeptide – amorphous calcium phosphate into a glass-ionomer cement. J. Dent. Res. 2003, 82, 914-918. |
| [291] | Uysal, T., Amasyali, M., Koyuturk, A.E., Sagdic, D. Efficiency of amorphous calcium phosphate-containing orthodontic composite and resin modified glass ionomer on demineralization evaluated by a new laser fluorescence device. Eur. J. Dent. 2009, 3, 127-134. |
| [292] | Reynolds, E.C. Anticariogenic complexes of amorphous calcium phosphate stabilized by casein phosphopeptides: a review. Spec. Care Dentist. 1998, 18, 8-16. |
| [293] | Tung, M.S., Eichmiller, F.C. Dental applications of amorphous calcium phosphates. J. Clinical Dentistry 1999, 10, 1-6. |
| [294] | Dorozhkin, S.V. Calcium orthophosphate-based biocomposites and hybrid biomaterials. J. Mater. Sci. 2009, 44, 2343-2387. |
| [295] | Uysal, T., Ustdal, A., Nur, M., Catalbas, B. Bond strength of ceramic brackets bonded to enamel with amorphous calcium phosphate-containing orthodontic composite. Eur. J. Orthodontics 2010, 32, 281-284. |
| [296] | Wei, D., Zhou, Y. Characteristic and biocompatibility of the TiO2-based coatings containing amorphous calcium phosphate before and after heat treatment. Appl. Surf. Sci. 2009, 255, 6232-6239. |
| [297] | Dunn, W.J. Shear bond strength of an amorphous calcium-phosphate-containing orthodontic resin cement. Am. J. Orthod. Dentofac. Orthoped. 2007, 131, 243-247. |
| [298] | Keçik, D., Çehreli, S.B., Şar, Ç., Ünver, B. Effect of acidulated phosphate fluoride and casein phosphopeptide-amorphous calcium phosphate application on shear bond strength of orthodontic brackets. Angle Orthod. 2008, 78, 129-133. |
| [299] | Foster, J.A., Berzins, D.W., Bradley, T.G. Bond strength of an amorphous calcium phosphate-containing orthodontic adhesive. Angle Orthod. 2008, 78, 339-344. |
| [300] | Uysal, T., Ulker, M., Akdogan, G., Ramoglu, S.I., Yilmaz, E. Bond strength of amorphous calcium phosphate-containing orthodontic composite used as a lingual retainer adhesive. Angle Orthod. 2009, 79, 117-121. |
| [301] | Uysal, T., Amasyali, M., Koyuturk, A.E., Ozcan, S., Sagdic, D. Amorphous calcium phosphate-containing orthodontic composites. Do they prevent demineralisation around orthodontic brackets? Austral. Orthodontic J. 2010, 26, 10-15. |
| [302] | Bröchner, A., Christensen, C., Kristensen, B., Tranæus, S., Karlsson, L., Sonnesen, L., Twetman, S. Treatment of post-orthodontic white spot lesions with casein phosphopeptide-stabilised amorphous calcium phosphate. Clin. Oral Invest. 2011, 15, 369-373. |
| [303] | Reynolds, E.C. Calcium phosphate-based remineralization systems: scientific evidence? Australian Dent. J. 2008, 53, 268-273. |
| [304] | Al Zraikat, H., Palamara, J.E., Messer, H.H., Burrow, M.F., Reynolds, E.C. The incorporation of casein phosphopeptide-amorphous calcium phosphate into a glass ionomer cement. Dent. Mater. 2011, 27, 235-243. |
| [305] | Beerens, M.W., van der Veen, M.H., van Beek, H., Ten Cate, J.M. Effects of casein phosphopeptide amorphous calcium fluoride phosphate paste on white spot lesions and dental plaque after orthodontic treatment: a 3-month follow-up. Eur. J. Oral Sci. 2010, 118, 610-617. |
| [306] | Zhao, J., Liu, Y., Sun, W.B., Zhang, H. Amorphous calcium phosphate and its application in dentistry. Chem. Cent. J. 2011, 8, 40 (7 pages). |
| [307] | Gupta, R., Prakash, V. CPP-ACP complex as a new adjunctive agent for remineralisation: a review. Oral Health Prev. Dent. 2011, 9, 151-165. |
| [308] | Zhang, Q., Zou, J., Yang, R., Zhou, X. Remineralization effects of casein phosphopeptide-amorphous calcium phosphate crème on artificial early enamel lesions of primary teeth. Int. J. Paediatric Dent. 2011, 21, 374-381. |
| [309] | Moreau, J.L., Sun, L., Chow, L.C., Xu, H.H.K. Mechanical and acid neutralizing properties and bacteria inhibition of amorphous calcium phosphate dental nanocomposite. J. Biomed. Mater. Res. B (Appl. Biomater.) 2011, 98B, 80-88. |
| [310] | Fletcher, J., Walsh, D., Fowler, C.E., Mann, S. Electrospun mats of PVP/ACP nanofibres for remineralization of enamel tooth surfaces. CrystEngComm 2011, 13, 3692-3697. |
| [311] | Uysal, T., Baysal, A., Uysal, B., Aydinbelge, M., Al-Qunaian, T. Do fluoride and casein phosphopeptide-amorphous calcium phosphate affect shear bond strength of orthodontic brackets bonded to a demineralized enamel surface? Angle Orthodontist 2011, 81, 490-495. |
| [312] | Tabrizi, A., Cakirer, B. A comparative evaluation of casein phosphopeptide-amorphous calcium phosphate and fluoride on the shear bond strength of orthodontic brackets. Eur. J. Orthodontics 2011, 33, 282-287. |
| [313] | Hamba, H., Nikaido, T., Inoue, G., Sadr, A., Tagami, J. Effects of CPP-ACP with sodium fluoride on inhibition of bovine enamel demineralization: a quantitative assessment using micro-computed tomography. J. Dent. 2011, 39, 405-413. |
| [314] | Ma, Z., Chen, F., Zhu, Y.J., Cui, T., Liu, X.Y. Amorphous calcium phosphate/poly(D,L-lactic acid) composite nanofibers: electrospinning preparation and biomineralization. J. Coll. Interf. Sci. 2011, 15, 371-379. |
| [315] | Kim, I., Kim, H.J., Kim, H.M. Array of amorphous calcium phosphate particles improves cellular activity on a hydrophobic surface. J. Biomed. Mater. Res. B (Appl. Biomater.) 2010, 93B, 113-121. |
| [316] | Sun, W., Zhang, F., Guo, J., Wu, J., Wu, W. Effects of amorphous calcium phosphate on periodontal ligament cell adhesion and proliferation in vitro. J. Medical and Biological Eng. 2008, 28, 107-112. |
| [317] | Llena, C., Forner, L., Baca, P. Anticariogenicity of casein phosphopeptide-amorphous calcium phosphate: a review of the literature. J. Contemp. Dent. Pract. 2009, 10, 1-9. |
| [318] | Cai, F., Shen, P., Morgan, M.V., Reynolds, E.C. Remineralization of enamel subsurface lesions in situ by sugar-free lozenges containing casein phosphopeptide-amorphous calcium phosphate. Australian Dental J. 2003, 48, 240-243. |
| [319] | Langhorst, S.E., O’Donnell, J.N.R., Skrtic, D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study. Dent. Mater. 2009, 25, 884-891. |
| [320] | Shen, P., Cai, F., Nowicki, A., Vincent, J., Reynolds, EC. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J. Dent. Res. 2001, 80, 2066-2070. |
| [321] | Iijima, Y., Cai, F., Shen, P., Walker, G., Reynolds, C., Reynolds, E.C. Acid resistance of enamel subsurface lesions remineralized by a sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. Caries Res. 2004, 38, 551-556. |
| [322] | Cai, F., Manton, D.J., Shen, P., Walker, G.D., Cross, K.J., Yuan, Y., Reynolds. C., Reynolds, E.C. Effect of addition of citric acid and casein phosphopeptide-amorphous calcium phosphate to a sugar-free chewing gum on enamel remineralization in situ. Caries Res. 2007, 41, 377-383. |
| [323] | Kumar, V.L.N., Itthagarun, A., King, N.M. The effect of casein phosphopeptide-amorphous calcium phosphate on remineralization of artificial caries-like lesions: an in vitro study. Australian Dental J. 2008, 53, 34-40. |
| [324] | Ranjitkar, S., Rodriguez, J.M., Kaidonis, J.A., Richards, L.C., Townsend, G.C., Bartlett, D.W. The effect of casein phosphopeptide-amorphous calcium phosphate on erosive enamel and dentine wear by toothbrush abrasion. J. Dentistry 2009, 37, 250-254. |
| [325] | Ranjitkar, S., Narayana, T., Kaidonis, J.A., Hughes, T.E., Richards, L.C., Townsend, G.C. The effect of casein phosphopeptide-amorphous calcium phosphate on erosive dentine wear. Australian Dental J. 2009, 54, 101-107. |
| [326] | Wegehaupt, F.J., Attin, T. The role of fluoride and casein phosphopeptide/amorphous calcium phosphate in the prevention of erosive/abrasive wear in an in vitro model using hydrochloric acid. Caries Res. 2010, 44, 358-363. |
| [327] | Al-Mullahi, A.M., Toumba, K.J. Effect of slow-release fluoride devices and casein phosphopeptide/amorphous calcium phosphate nanocomplexes on enamel remineralization in vitro. Caries Res. 2010, 44, 364-371. |
| [328] | Giniger, M., MacDonald, J., Spaid, M., Felix, H. A 180-day clinical investigation of the tooth whitening efficacy of a bleaching gel with added amorphous calcium phosphate. J. Clinical Dentistry 2005, 16, 11-16. |
| [329] | Giniger, M., MacDonald, J., Ziemba, S., Felix, H. The clinical performance of professionally dispensed bleaching gel with added amorphous calcium phosphate. J. Am. Dental Association 2005, 136, 383-392. |
| [330] | Ramalingam, L., Messer, L.B., Reynolds, E.C. Adding casein phosphopeptide-amorphous calcium phosphate to sports drinks to eliminate in vitro erosion. Pediatric Dentistry 2005, 27, 61-67. |
| [331] | Panich, M., Poolthong, S. The effect of casein phosphopeptide-amorphous calcium phosphate and a cola soft drink on in vitro enamel hardness. J. Am. Dental Assoc. 2009, 140, 455-460. |
| [332] | Silva, K.G., Pedrini, D., Delbem, A.C.B., Ferreira, L., Cannon, M. In situ evaluation of the remineralizing capacity of pit and fissure sealants containing amorphous calcium phosphate and/or fluoride. Acta Odontologica Scandinavica 2010, 68, 11-18. |
| [333] | Bayrak, S., Tunc, E.S., Sonmez, I.S., Egilmez, T., Ozmen, B. Effects of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) application on enamel microhardness after bleaching. Am. J. Dentistry 2009, 22, 393-396. |
| [334] | Yengopal, V., Mickenautsch, S. Caries preventive effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP): a meta-analysis. Acta Odontologica Scandinavica 2009, 67, 321-332. |
| [335] | Walker, G.D., Cai, F., Shen, P., Reynolds, C., Ward, B., Fone, C., Honda, S., Koganei, M., Oda, M., Reynolds, E.C. Increased remineralization of tooth enamel by milk containing added casein phosphopeptide-amorphous calcium phosphate. J. Dairy Res. 2006, 73, 74-78. |
| [336] | Walker, G.D., Cai, F., Shen, P., Bailey, D.L., Yuan, Y., Cochrane, N.J., Reynolds, C., Reynolds, E.C. Consumption of milk with added casein phosphopeptide-amorphous calcium phosphate remineralizes enamel subsurface lesions in situ. Australian Dental J. 2009, 54, 245-249. |
| [337] | Willershausen, B., Schulz-Dobrick, B., Gleissner, C. In vitro evaluation of enamel remineralisation by a casein phosphopeptide-amorphous calcium phosphate paste. Oral Health & Preventive Dentistry 2009, 7, 13-21. |
| [338] | Mei, H.L., Chen, L.Y., Zhang, D., Zhang, P.L., Liu, B., Zhao, W., Qi, H.Y. Effects of casein phosphopeptide-stabilized amorphous calcium phosphate solution on enamel remineralization. J. Clin. Rehabil. Tiss. Eng. Res. 2009, 13, 4825-4828. |
| [339] | Shinohara, M., Uchida, K., Shimada, S., Tomioka, K., Suzuki, N., Minegishi, T., Kawahashi, S., Yoshikawa, Y., Ohashi, N. Novel concentration method for the detection of norovirus and sapovirus from water using minute particles of amorphous calcium phosphate. J. Medical Microbiology 2011, 60, 780-786. |
| [340] | Dorozhkin, S.V. Nanodimensional and nanocrystalline apatites and other calcium orthophosphates in biomedical engineering, biology and medicine. Materials 2009, 2, 1975-2045. |
| [341] | Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715-734. |
| [342] | Suvorova, E.I., Buffat, P.A. Size effect in X-ray and electron diffraction patterns from hydroxyapatite particles. Crystallogr. Rep. 2001, 46, 722-729. |
| [343] | Suvorova, E.I., Buffat, P.A. Electron diffraction and high resolution transmission electron microscopy in the characterization of calcium phosphate precipitation from aqueous solutions under biomineralization conditions. Eur. Cell Mater. 2001, 1, 27-42. |
| [344] | Celotti, G., Tampieri, A., Sprio, S., Landi, E., Bertinetti, L., Martra, G., Ducati. C. Crystallinity in apatites: how can a truly disordered fraction be distinguished from nanosize crystalline domains? J. Mater. Sci. Mater. Med. 2006, 17, 1079-1087. |
| [345] | Tadic, D., Beckmann, F., Schwarz, K., Epple, M. A novel method to produce hydroxyapatite objects with interconnecting porosity that avoids sintering. Biomaterials 2004, 25, 3335-3340. |
| [346] | Chandanshive, B., Dyondi, D., Ajgaonkar, V.R., Banerjee, R., Khushalani, D. Biocompatible calcium phosphate based tubes. J. Mater. Chem. 2010, 20, 6923-6928. |
| [347] | Scott, P.R., Crow, J.A., LeGeros, R.Z., Kruger, M.B. A pressure-induced amorphous phase transition in magnesium-substituted β-tricalcium phosphate. Solid State Comm. 2011, 151, 1609-1611. |
| [348] | Xu, H.H.K., Moreau, J.L., Sun, L., Chow, L.C. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011, 27, 762-769. |
| [349] | Gross, K.A., Young, C.J., Beck, M.A., Keebaugh, E.W., Bronts, T.J., Saber-Samandari, S., Riley, D.P. Characterization and dissolution of functionalized amorphous calcium phosphate biolayers using single-splat technology. Acta Biomater. 2011, 7, 2270-2275. |
| [350] | Dosen, A., Giese, R.F. Thermal decomposition of brushite, CaHPO4·2H2O to monetite CaHPO4 and the formation of an amorphous phase. Am. Mineralogist 2011, 96, 368-373. |

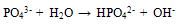
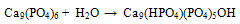
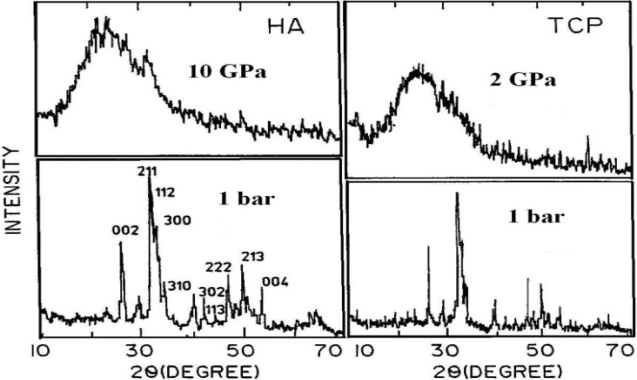
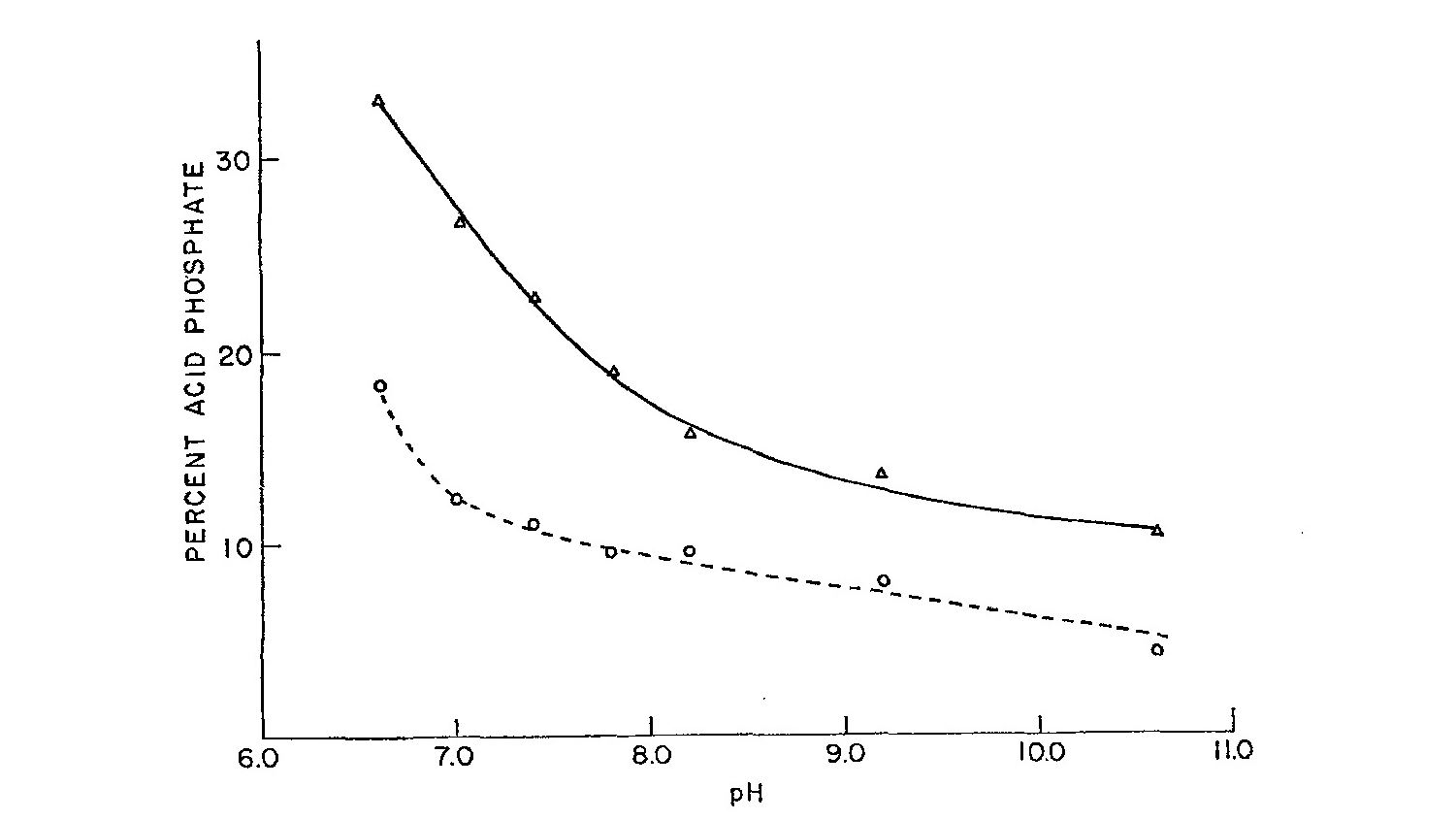
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML