-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2012; 2(1): 16-18
doi: 10.5923/j.ijmc.20120201.03
Solubility and Solvation Parameters of Barium Sulphate in Mixed Ethanol-Water Mixtures at 301.15 K
Esam A. Gomaa
Chemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt
Correspondence to: Esam A. Gomaa , Chemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The molar solubility of barium sulphate (BS) in mixed ethanol (EtOH)-water solvents was measured at 301.15 K . From the molar solubilities, the solvation parameters, activity coefficients, solubility products, free energies of solvation and transfer free energies for interaction of (BS) from water as reference solvent to mixed (EtOH-H2O) solvents were evaluated. The different volumes for (BS) like, molar , Van der Waals and electrostriction volumes were evaluated. All the different volumes for (BS) in mixed EtOH-H2O solvents indicate that the volumes increase by more adding alcohol favouring more energy required for solvation .The solvation free energy values were also discussed. This work explains two methodsused for the determination of(BS) concentrations in solutions and water, These two methods are volume determination and conductivity of the salt under consideration.This because barium salts are important salts responsible for the hardenss of water.
Keywords: Molar Solubility, Barium Sulphate, Free Energies of Solvation, Water- Ethanol Solvents
Article Outline
1. Introduction
- The solubility of any electrolyte in solvents depends on the properties for both solte and solvent.Debye-Huckel recognizes solvents by their bulk properties, namely relative permittivity, viscosity and density of pure solvent.Also the bulk properties of the solvents decrease as the electrolyte concentration increase. On the molecular scale solvents may be classified according to hard and soft donor and acceptor properties of both solvent and solute[1]. Interactions of solvent and solute depend on the electron distribution between donor and acceptor atoms in these substances. Theoretical approaches on Born-Oppenheimer (BO) level take into consideration internal charge distributions of solvent molecules with the help of molecular properties such as partial charges, dipole, quadrupole, higher n-pole moments, polarizabilities, etc. McMillan –Mayer (MM) approches by takingaccount of them intheir potential mean force. Chemical models (CM) on these levels yield importent progress as compared to the classical Debye-Huckel theory with its exclusive columbic, ion-ion interactions in a dielectric medium[2]. Applied solution chemistry successfully uses empirical parameters to describe a solvent’s ability to interact with acceptors such as protons, cations or Lewis acids. Examples of these are Guttmann’s donor number DN and Kemlet and Taft’s B parameter. The ability of a solvent to interact with donor’s is commonly characterizes by Dimroth and Richard’s ET parameter. Mayer Gutmann and Gorger’s acceptor number AN or Taft and Kamlets α parameter. The main parmeter used for the assessment of the polarity and polarizability of a solvent is solvatochromic parameter π*. A commonly used rough classification distinguishes between protic, aporotic and inert solvents. We adopt this classification taking into account the acid- base properties, polarity and polarizability.On the other hand the solutes can be classified to ionophores or ionogens according to their ions in solutions. Ionophores are substances which in their pure state exist as ionic crystal, where their ions already exist as the component particles within their structure. Dissociation of the ionic crystal yield a first step the solvated cations and anions followed by the formation of solvatedion pairs. Symmetrical electrolytes, associate together to form neutral ion pairs, whereas the ion pairs from unsymmetrical electrolytes are charged. Ionogenes are substances which form ions through chemical reactions, either with solvent molecules or with suitable added species in the solution. In their pure states at the temperature and pressure of investgation, they exist as neutral molecules[3].The solubility of solutes in mixed solvents is of great practical importance since many industrial process as well as laboratory procedures call for the use of solvent mixtures[4]. The solubility of solutes in mixed solvents depends primarily on the solvation of solutes or their constituent ions by the components of solvent mixtures[4]. Studying the thermodynamics of different salts, is important for evaluating the single ion thermodynamic parameters which help in explain the preferential solvation of the ions[5].Removal of sulphate ions from an alkaline medium using solvent extraction was very important to get rid of these hand ions[3]. The aim of the work is to estimate the solubilities of (BS) in the mixed EtOH-H2O solvents and its solvation parameters. Several treatment methods have been developed to reduce high sulphate concentration from different waste water, i.e, the limestone process, the barium salt process, the cost-effective sulphate removal and Saving process. The barium process is the best as described by precipitation of sulphate as barium sulphate.H2S and BaS as by product for this last process can be removed by stripping with CO2 and the barium carbonate is obtained which is the stating material. Therefore barium carbonate is recycled[6]. Giving date about the solubilities and solvation of (BS) can help us in future studies and applications for water purification.
2. Experimental
2.1. Materials
- The used barium sulphate (BS) and ethanol (EtOH) were supplied from Merck CO. The saturated solution of barium sulphate (BS) was prepared by dissolving little solid amount in closed test tubes containing different EtOH-H2O mixtures. The mixtures were then saturated with N2 gas as inert atmosphere. The tubes were placed in a shaking thermostat (Model GEL) for a period of one week till equilibrium reached.
2.2. Solubility Measurements
- The solubility of BS in each mixture were measured conductometrically exact (three minimum times) by using conductometer of the type YSI model-35 and it was connected with an ultra-thermostat of the type Kottermann-4130. All conductances were measured at 301.15 K. The conductivity was easily measured, reproducible and also cheap method. The conductance values were measured directly without waiting, i.e consuming time. The deviation in the experimental conductance’s are very small .Since the solubility of (BS) is sparingly ones, therefore conductivity is very good method for the analytical evaluations. The accuracy of the solubility data is in average of third number after coma, plus or minus as cited in previous work[7].
3. Results and Discussion
3.1. Solubility Results
- The molar solubilities for barium sulphate (BS) at 301.15 K were measured conductometrically and the 10 g values are cited in Table 1 in water, ethanol (EtOH) and their mixtures. The solubility of (BS) in water agreed well with that in Literature[8]. The activity coefficients were calculated by the use of Debye-Hückel equation [9].
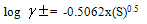 | (1) |
 | (2) |
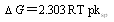 | (3) |
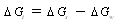 | (4) |
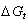 increase in positivity by increasing the mole fraction of EtOH in the mixtures. This is due to more difficult solvation in the mixed solvents than that of water. Polar solvents like H2O or EtOH or their mixtures can not penetrate (BS) lattice due to the very high heats of formation, free energy, entropy and heat capacity for the salt[10].Ethanol is muck less polar than water. Since (BS) is insoluble in water. Therefore (BS) would be even less soluble in ethanol.According to this small solubility, applying some thermodynamic model was done to explain the precipitation of (BS) [11].
increase in positivity by increasing the mole fraction of EtOH in the mixtures. This is due to more difficult solvation in the mixed solvents than that of water. Polar solvents like H2O or EtOH or their mixtures can not penetrate (BS) lattice due to the very high heats of formation, free energy, entropy and heat capacity for the salt[10].Ethanol is muck less polar than water. Since (BS) is insoluble in water. Therefore (BS) would be even less soluble in ethanol.According to this small solubility, applying some thermodynamic model was done to explain the precipitation of (BS) [11].
|
|
3.2. Volumes Results
- The molar volumes (VM or VM) for (BS) in mixed EtOH-H2O were calculated by dividing the molecular weight by the exact solution densities and their values are tabulated in Table (3). The packing density (P) as represented by Kim (in ref. 9), i.e., the relation between Van der Waals volume (Vw) and the molar volume for relatively large molecules was found to be constant and equal 0.661.
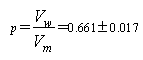 | (5) |
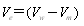 | (6) |
4. Conclusions
- This work provide an analyst a lot of data which help hem in the determination of (BS) in solutions, Since the solubility of barium sulphate is very low, therefore volume measurement can help for the analytical evaluation.
References
| [1] | R. A. Robinson and R .H. Stokes, “Electrolytes solutions”., Butterworth & Co. (Pulishers) Ltd,Newton Abbot,Devon (2002). |
| [2] | J.M.G.Barthel, H.Krienke and W. Kunz,: Physical Chemistry of electrolyte solutions “Springer – Verlag, Darmstadt, New York (1998). |
| [3] | Trevor m. Letcher,: Deveolpments and applications in solubility”. The Royal Society of Chemistry, Cambridge,(2007). |
| [4] | Yizkak Marcus, "Solubility and solvation in mixed solvent systems", pure and Applied Chem., 62 (1990) 2069-2076. |
| [5] | Esam A. Gomaa, "Single ion free energies of some ion and the hydrophobic interactions of Ph4 AsBPh4 and Ph4SbB Ph4 in mixed ethanol-water solvents". Thermochimica Acta, 156 (1989) 91-99. |
| [6] | Cleophase Ngoie Mpinga, "Removal of aluminium and sulphate ions from alkaline medium using solvent extraction., Master of Technology, Faculty of Engineering, Cape Peninsula University of Technology (2009). |
| [7] | E. A. Gomaa, "Solvation parameters of lead acetate in mixed water-N, N-dimethylformamide mixtures at 298.15 K ". Analele Universitätii den Bucuresti, 19, 1 (2010) 45-48. |
| [8] | Perry's "Chemical Engineering Handbook, section 2, Physical and Chemical data, 8th edition”, McGraw Hill., USA (2008). |
| [9] | A. A. El-Khouly, Essam A. Gomaa and S Abou El-Leef, "Conductometry and solubility study of Cd2 Kryptofix-22) complexes in various hydroorganic solvents", Bulletin of Electrochemistry, 19(4), (2003) 153-164. |
| [10] | Jim Plambec la @ Malberta. Ca. |
| [11] | L. Vicum, M. Hazzotti and J. Baldyga, "Applying a thermodynamic model to the non-stoichiometric precipitation of barium sulphate". Chemical Engineering and Technology, 26(2003) 352-333. |
| [12] | Gy.Takli,”Evaluation of individual ionic partial molar volumes in aqueous solutions” J.Chem. Thermodynamics, 40(2008)770-776. |
| [13] | Yizhak Marcus,”On the preferential solvation of drugs and PAHs in binary solvent mixtures”,Journal Molecular Liquids,140(2008)61-67. |
| [14] | K.Rajagopal,S.Edwin Gladson,” Partial molar and partial compressibility of four homologous alpha amino acids in aqueous sodium fluoride solutions at different temperatures.”, J.Chem.Thermodynamics,43(2011)852-867. |
| [15] | Ewa Kamenska-Piotrowice,Janusz Stangret,Joanna Szymanska-Cybulska,” Solvation of cobalt(II) and trifluoromethanesulfonate ions in N,N-dimethylformamide-methanol mixed solvents,studied by means of FT-IR spectroscopy.”,Spectrochimica Acta,Part A,60(2007), 1-8. |
| [16] | E.A.Gomaa and B.M.Al-Jahdali,”Association of Cu(NO3)2 eith Kryptofix-221 in mixed (MeOH-DMF) solvents at different temperatures.”,American Journal of Fluid Dynamics, 1(1),2011,4-8. |
| [17] | Nagah A.El-Shishtawi,Maany a.Hammada and Esam A.Gomaa,” Inflence of peranent magnet on the association constants of FeCl3 + 10% PVA(polyvinyl alcohol)in 50% ethanol-water solutions conductometrically at 298.15K using new equation for 1:3 asymmetric electrolytes.”, Physical Chemistry, 1(1) ,2011, 14-16. |
| [18] | E.A.Gomaa,”Solvation parameters of lead acetate in mixed, N,N-dimethylformamide–water mixtures at 298.15K.”, Analele Universitate din Bucuresti-Chimie, vol.19, (2010) 45-48. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML